Should you wait for a better vaccine?
It’s being touted as our ticket back to normal life, now an expert has answered all the key questions about Australia’s vaccine rollout.
Vaccinating against COVID-19 is the easiest way for Australians to get their normal lives back, but millions are hesitant to get the jab.
News.com.au’s Our Best Shot campaign answers your questions about the COVID-19 vaccine roll out.
We’ll debunk myths about vaccines, answer your concerns about the jab and tell you when you can get the COVID-19 vaccine.
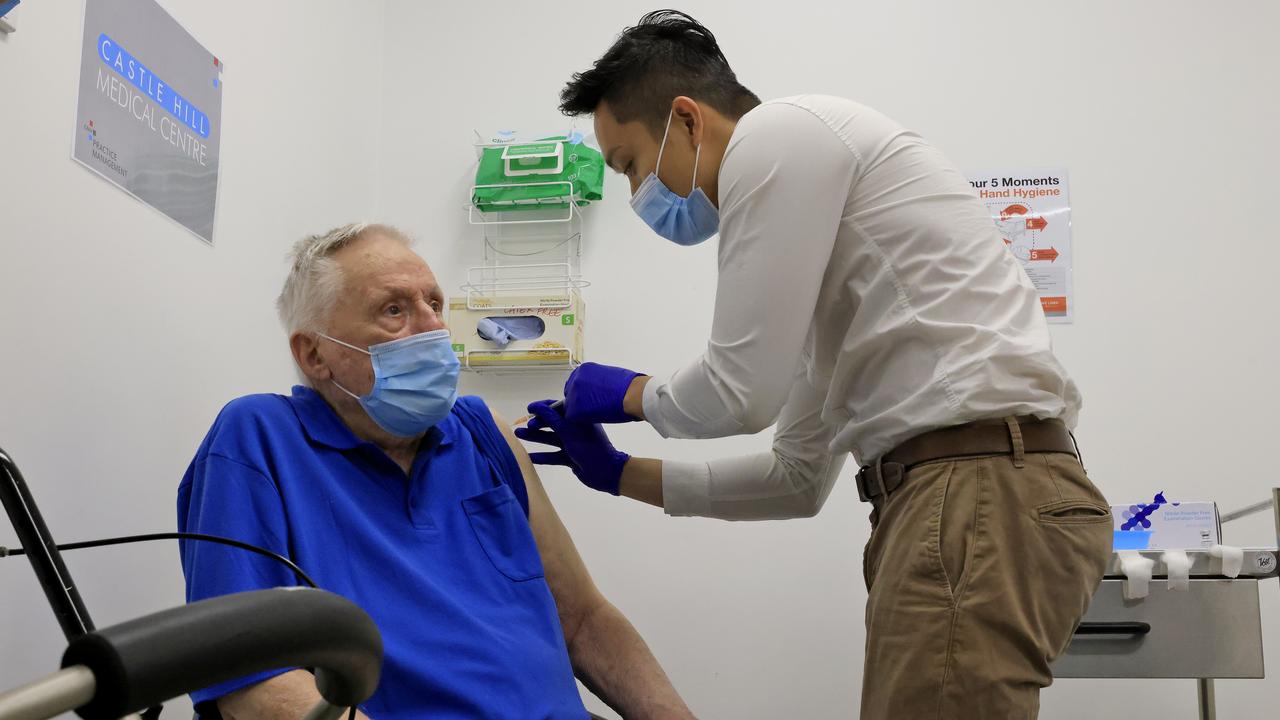
The COVID-19 vaccine has arrived in Australia and is being rolled out across the country. More than 20 million Australians – everyone over the age of 16, excluding pregnant women – will be offered a COVID vaccine in 2021.
But even as we finally reach a point where we can see an end to our profoundly altered way of life, there is real resistance out in the community towards getting vaccinated at all.
Anti-vaxxers held rallies across the country over the weekend, and footage of people booing the mere mention of the vaccine at the Australian Open has gone viral around the world.
As an immunologist, I have spent 30 years caring for patients with weak immune systems and immune diseases.
I have seen the devastating effects that COVID-19 misinformation has had on my patients’ health.
Some stopped lifesaving treatments for their immune diseases, resulting in serious relapses. Others missed appointments and treatments because they were afraid that a hospital visit would result in them contracting COVID.
And some couldn’t obtain hydroxychloroquine, which is sometimes used to treat immune diseases, because people in the media claimed – without proof – that it could prevent or treat COVID.
Medical and vaccine misinformation is not new, and has devastating effects. Diseases that were once effectively contained, like measles and polio, have found new purchase in communities where vaccination rates are low.
Fearmongering about vaccines has led to increased stigma against people with autism. And in the age of social media, it spreads faster and more dangerously than ever.
Even federal politicians have recently made headlines for spreading false and harmful information online.

The questions patients ask me at my clinics suggest the efforts of governments and health authorities to educate the public are leaving a lot of people confused and searching for further information and reassurance – often in the wrong places.
Misinformation cannot be countered with strident language or finger-wagging. The way forward is with data, presented in simple language, and with a calm voice.
The media should give airtime to those who inform and explain, rather than to those who shriek or grandstand.
With that in mind, here are some common questions I encounter, and what I tell the patients who ask them.
If you have more questions and don’t have an immunologist on tap, please ask your local doctor.
DO YOU RECOMMEND THE COVID-19 VACCINATION?
Yes. I will definitely be vaccinated, hopefully within the next month, as will my daughter, who also works in a hospital. My wife and sons are also keen, but understand that they must wait in line. The only people who should probably not be vaccinated (yet) are pregnant women and children under 16, as safety data for these groups is not in.
ARE THE VACCINES SAFE?
The safety of the COVID vaccines is similar to that of other vaccines. In the Pfizer safety trial, which included over 40,000 people, just 0.6 per cent of participants who received the vaccine reported a “serious safety event” – none of which were assessed as due to the vaccine. The most common symptoms were pain at the injection site, followed by fatigue and a headache. By comparison, 0.5 per cent of participants who received a placebo vaccine reported a serious safety event.
CAN THE VACCINE GIVE ME COVID-19?
No. The vaccines are synthetic; they don’t contain the whole COVID virus. The vaccines result in your cells making just one COVID protein, which cannot turn into the whole virus.
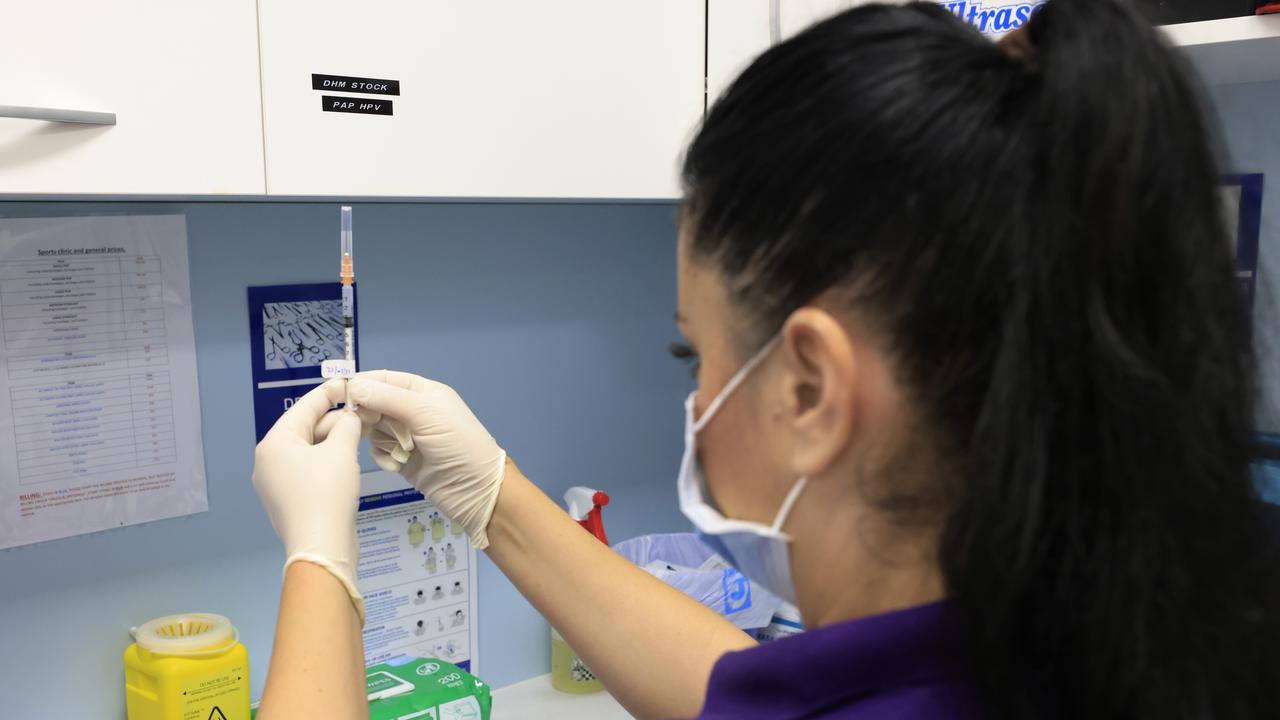
BUT DOESN’T THE VACCINE’S RAPID APPROVAL MEAN ITS SAFETY WAS NOT ASSESSED PROPERLY?
The quality of the studies was not compromised at all. What was shortened was the time to get the vaccine trials started, the time between one study finishing and the next starting, and the time regulators took with the paperwork.
Whether approval is fast or slow, rare safety concerns only come to light once a vaccine or drug is administered to many more individuals. For example, severe allergy (anaphylaxis) to the Pfizer vaccine was not seen in clinical trials, but has now been recognised and its risk estimated at about 1 in 200,000 doses. So we should anticipate 50 such reactions if 10 million Australians receive this vaccine.
WILL I NEED TO BE VACCINATED AGAIN?
I suspect so, for two reasons. First, the virus is prone to mutating, so it may eventually escape the effects of existing vaccines. But we have successfully addressed that issue with other viruses like influenza, where a new vaccine is developed regularly.
Second, many vaccines lose effectiveness over time. For some, like hepatitis A and B, that may take decades. For others, like influenza, that can be months. For COVID, we just don’t know yet, but ongoing follow-up will tell us soon enough. Don’t be surprised if we end up getting a COVID vaccine once a year, like the flu.
WHY AM I GETTING VACCINATED IF THERE ARE SO FEW COVID-19 INFECTIONS IN AUSTRALIA?
All Australians stand to gain from vaccination. It will protect those for whom the vaccine never worked or has stopped working; allow society to reopen and stay open; allow people to get their jobs and incomes back; and allow us to travel, at first interstate and eventually overseas.
WILL THE GOVERNMENT FORCE ME TO BE VACCINATED?
I doubt it. Persuasion is always better in health. If I tell patients that they must take a medicine, but they don’t want to, they will silently take the prescription and never come back.
I THINK I AM HIGHER RISK, SO I NEED THE VACCINE EARLIER THAN MOST. HOW WILL THE GOVERNMENT KNOW THIS?
No idea! This has not been communicated by governments yet. I suggest you let your GP know, and ask them if their clinic will be a vaccine site.
SHOULD I WAIT FOR A BETTER VACCINE?
That partly depends on the risk to you and those around you – for example, if you have contact with those who are older, are pregnant, or have an underlying illness.
But if everyone waits, then Australia remains at risk. It is likely that COVID vaccines will improve over time. But it took decades for some vaccines to be optimised.
If I had told my HIV-positive patients in 1990 to wait 20 years for the better drugs that eventually did arrive, then most of those patients would be dead. For now, the glass is half-full, and that’s better than empty.
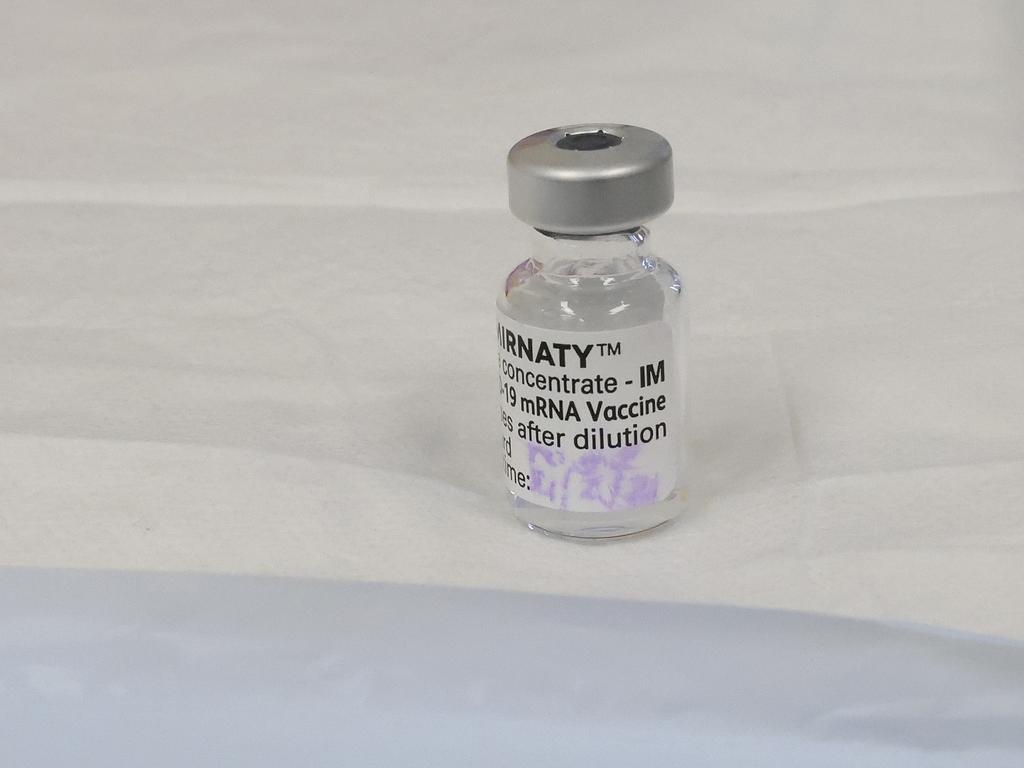
HOW WILL I KNOW IF THE VACCINE HAS WORKED?
You probably won’t, in the same way we never “know” whether most of our childhood vaccines worked. A blood test that would let us know would be very helpful, particularly for those who are at greater risk of exposure.
Professor Andrew Carr graduated from the University of New South Wales in 1984, and completed his doctoral thesis on HIV-related drug hypersensitivity in 1994. He is a clinical immunologist and immunopathologist, Director of the Immunology and HIV Unit at St Vincent’s Hospital, Sydney, and Professor of Medicine at UNSW. He has authored more than 350 peer-reviewed publications, mostly on the complications of HIV medications.
References
Wakefield AJ, Murch SH, Anthony A, et al. Ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children. Lancet 1998; 351: 637-41. doi: 10.1016/s0140-6736 (97) 11096-0.
Begg C, Cho M, Eastwood S, et al. Improving the quality of reporting of randomised controlled trials. The CONSORT statement. JAMA 1996; 276: 637-9. doi: 10.1001/jama.276.8.637.
Guyatt GH, Oxman AD, Vist GE, et al; GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008; 336: 924-26. doi: 10.1136/bmj.39489.470347.AD.
Baden LR, El Sahly HM, Essink B, et al; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021; 384: 403-16. doi: 10.1056/NEJMoa2035389.
Voysey M, Costa Clemens SA, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa and the UK. Lancet 2020; Published online December 8, 2020. doi.org/10.1016/S0140-6736 (20) 32661-1.
Shimabukuro TT, Cole M, Su JR. Reports of anaphylaxis after receipt of mRNA COVID-19 vaccines in the US – December 14, 2020-January 18, 2021. JAMA 2021 (Published online February 12, 2021). doi: 10.1001/jama.2021.1967.
National COVID-19 Clinical Evidence Taskforce. Clinical care guidelines. https://covid19evidence.net.au/ (accessed 17 February, 2021).
World Health Organisation. Clinical management of COVID-19. Available at: https://www.who.int/publications/i/item/clinical-management-of-covid-19 (accessed 17 February, 2021).
Australian Government Department of Health. COVID-19 vaccines. Available at: https://www.health.gov.au/initiatives-and-programs/covid-19-vaccines (accessed 17 February,
2021).