Expert reveals why coronavirus vaccine approval in Australia is taking so long
Aussies are getting impatient for a COVID vaccine but one expert has explained why we’re waiting while the rest of the world moves forward.

As coronavirus outbreaks in Sydney and Melbourne grow, some are calling for the vaccination program to be brought forward. But one expert has explained why authorities want to wait until March.
The Morrison Government has confirmed Australia won’t begin its vaccination program until after it has been approved by the Therapeutic Goods Administration (TGA) and the rollout won’t begin until March.
But other countries including Britain, the United States and the European Union have already begun vaccinating their citizens as they battle a dramatic increase in infections.
On January 1, the World Health Organisation (WHO) approved the Pfizer/BioNTech vaccine for emergency use to accelerate vaccine programs in the developing world.
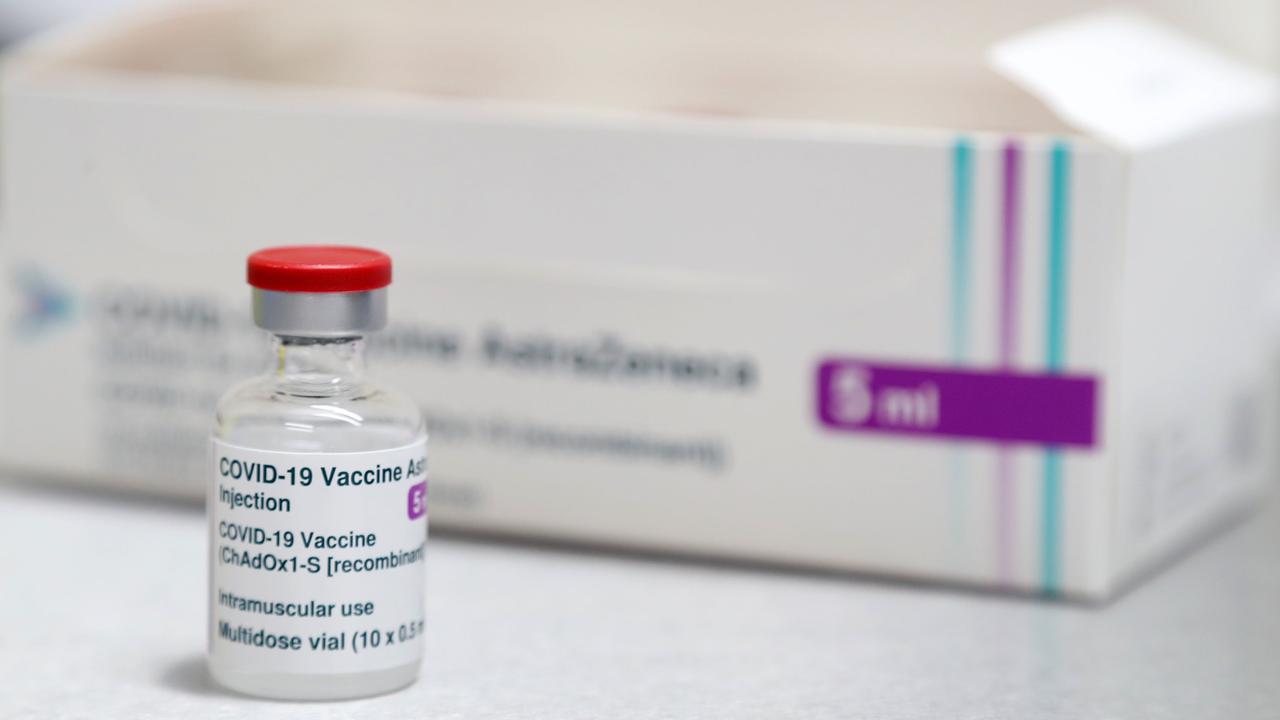
Phase III trials involving up to 40,000 people for at least three vaccines have proven to be safe. Australia has deals in place to get the Pfizer/BioNTech vaccine as well as the University of Oxford/AstraZeneca vaccine. Another vaccine from Novavax may be available later in the year.
Britain, which is battling more than 50,000 new cases a day, was the first to approve the use of the Pfizer vaccine last year and began its roll out of the Oxford University version this week. Its expected to finish vaccinating priority groups by mid-January.
Labor leader Anthony Albanese has said the Government had “dropped the ball” when it comes to the coronavirus vaccine.
“Even if the Pfizer and AstraZeneca vaccines are approved this month, the Government has said Australians won’t get access to them until March,” Mr Albanese tweeted.
“That’s clearly not good enough.”
He said there was only one reason for that.
“Other countries started securing vaccine deals in March and April last year, but the Morrison Government didn’t secure our first deal until September,” he wrote.
“We need a government that isn’t lagging behind the rest of the world, and a government that doesn’t use a global pandemic for more spin and little follow up.”
He said a timely vaccine was crucial in helping Australia get back to COVID normal.
Other experts have also suggested the faster rollout of the vaccination program among those most vulnerable should be debated.
RELATED: Britain shut down as virus crises worsens
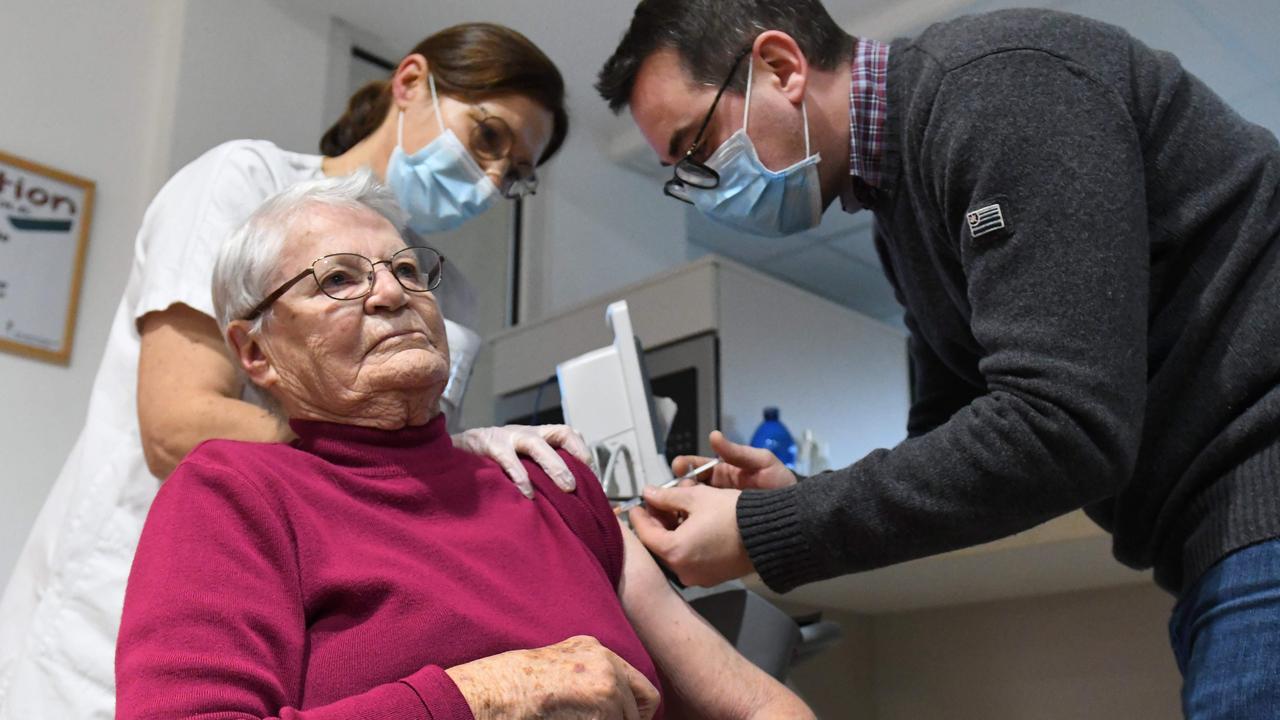
“The vaccines are a result of an incredible international scientific effort and are our only hope at beginning our “post-COVID normal”, the quicker the better,” Adjunct Professor Heidi Drummer of the Burnet Institute wrote in the Sydney Morning Herald.
“If the TGA would move more rapidly, we could bring forward Australia’s vaccine timetable with confidence.”
However, Prime Minister Scott Morrison told 3AW on Tuesday the safety processes in Australia didn’t just end when the TGA approved the vaccine.
He said individual batches of vaccine also needed to be tested before being distributed to the population.
“Suggestions that I’ve heard about trying to rush this process I think can be very dangerous.
“I don’t think Australians would want us just willy nilly sending out vials of vaccine that haven’t had their batch tested, which is the normal process that occurs with any TGA-approved vaccine.”
Mr Morrison said the UK was rolling out the vaccine on an emergency basis and it was his understanding authorities were not testing batches of the vaccine before they were sent out for use.
“Australia is not in an emergency situation, like the UK, so we don’t have to cut corners, we don’t have to take unnecessary risks and we’re learning a lot from some of the issues that are presenting themselves and the confusion in particular, that is there is about doses and distribution and administering of the vaccine.”
Professor Allen Cheng, who is chair of an advisory committee for vaccines that’s providing expert advice to the TGA, also explained more thoroughly in a Twitter thread what’s involved in vaccine regulation.
RELATED: PM slams Albanese’s ‘dangerous’ virus demand
SERIOUS SIDE EFFECTS
Prof Cheng said vaccines must first be proved to be safe and effective at preventing the disease.
“The phase 3 studies have generally included about 20,000-25,000 people who received the vaccine,” Prof Cheng wrote.
“This is sufficient to exclude moderately uncommon side effects, but not serious but rare side effects (eg those occurring in less than 1 in 10,000 people).”
He said it could be difficult to work out whether a serious side effect reported in one person was just chance or something to worry about.
Prof Cheng pointed to the three cases of transverse myelitis (spinal cord inflammation) that were reported in the AstraZeneca trial. One happened 14 days after the person got the vaccine, another case was thought to be due to pre-existing multiple sclerosis and there was a third case in the non-vaccine group.
There were also cases of anaphylaxis in the UK and US that were only picked up later when people with anaphylaxis had severe allergic reactions after getting the Pfizer vaccine. Those with severe allergies are now being advised not to get the vaccine.
RELATED: How do Pfizer and Moderna vaccines work?
A few comments on endpoints for COVID vaccine trials. For regulators, the main question when considering effectiveness is whether the vaccine reduces the risk of symptomatic COVID. https://t.co/NTcsGGVMUa
— Allen Cheng (@peripatetical) December 20, 2020

BATCH TESTING OF VACCINES
Batch testing of vaccines to ensure they are of sufficient quality is also important.
“We want to know that there is the correct amount of vaccine in each dose,” he wrote.
“We want to know they are free from contamination. That there are no differences between different batches or those made in different factories. We need to know the shelf life under different conditions.”
He said there were questions about whether the vaccines could be safely used in pregnant or breastfeeding women, and whether they could be given together with other vaccines such as the flu vaccine.
“For COVID vaccines, we have published papers that report that the vaccines appear to be effective and generally safe,” he noted.
“Many people think that published papers are the gold standard in evidence, but they just scrape the surface of what we want to know.”
Regulatory submissions contain much more information, Prof Cheng noted, adding that they run to tens of thousands of pages.
“I’ve read ones in the past that were more than 100,000 pages,” he wrote.
“Ultimately, the question is whether the benefit of using the vaccine outweighs the known risks and the uncertainties.
“Countries where there are hundreds or thousands of deaths each day are clearly willing to tolerate some uncertainty to prevent this, and this is appropriate,” he said.
However, Prof Cheng noted that some agencies including the US Food and Drug Administration had only issued “temporary” approvals or “emergency use authorisation” reflecting that not all the data is available yet.
“We’re in a different position in Australia – even with the current situation in NSW and Victoria, we can afford to wait for the TGA to do its job and make sure we’re getting a safe, effective and quality vaccine.”




