Coronavirus Australia: Health officer’s new warning after AstraZeneca vaccine clot case
Vaccine experts have issued a warning after a 44-year-old man was taken to hospital with a rare blood clot disorder days after getting his jab.

A Victorian man has been admitted to hospital with a rare blood clotting disorder just a few days after receiving the AstraZeneca vaccine.
Acting Chief Medical Officer Professor Michael Kidd confirmed the Therapeutic Goods Administration was urgently investigating the reported case after the man presented to Melbourne’s Box Hill Hospital on Good Friday with fever and abdominal pain.
The man received his vaccine dose on March 22.
He was found to have abdominal clots with a very low platelet count when he arrived at hospital.

Prof Kidd said it was not yet clear whether the condition was related to the vaccination, following reports of similar instances emerging in European countries and Canada.
He said health authorities were taking Australia’s case “very seriously”.
“Cerebral venous thrombosis is a very rare disorder that has previously not been known to be associated with vaccination, however it has been noted as a complication of people who have contracted COVID-19,” Prof Kidd said.
“No cases of central venous sinus thrombosis have been reported in Australia to date, in the time period of concern following vaccination, which is within four to 20 days.
“The TGA has received only one report of a case of thrombosis and thrombocytopenia following vaccination with the AstraZeneca vaccine in Australia but the causal link has not yet been established.”
Despite this, a new warning has been issued for vaccine patients from the Australian Technical Advisory Group on Immunisation (ATAGI), advising them of the risk.
Prof Kidd advised healthcare workers to be aware of the condition.
“This condition has presented as either a clot appearing in the brain or as thrombosis in other sites, including in the intra-abdominal venous systems,” he said.
“If cerebral venous sinus thrombosis or another severe thrombotic case is suspected in a patient who has received a COVID-19 vaccine, please refer them to an emergency department for further urgent assessment and haematology consultation.”
Prof Kidd said people who received either of the COVID-19 vaccines should be aware of the common side effects, which include fever, sore muscles, tiredness and headaches usually 24 hours after the dose.
“The reports from overseas of rare clotting disorders have occurred later than this. Between day four and day 20, after vaccination, and have generally caused severe symptoms requiring hospitalisation,” he said.
“People should be particularly alert to severe persistent headaches occurring 4- 20 days after vaccination and which are different to the usual pattern of headaches that people may experience at other times and which do not settle with paracetamol or other over the counter painkillers.”

750,000 doses of COVID-19 vaccines have been administered across the country as of midday Thursday.
The World Health Organisation and the EU’s health watchdog have both deemed the AstraZeneca vaccine safe after fears were raised of the jab having serious side effects.
Germany recently announced they were restricting vaccine doses for people under 65 after reports of people with “very rare but very serious cases of thrombosis (blood clots)”.
In March, the European Medicines Agency (EMA) said the shot was not linked to an increased risk of blood clots.
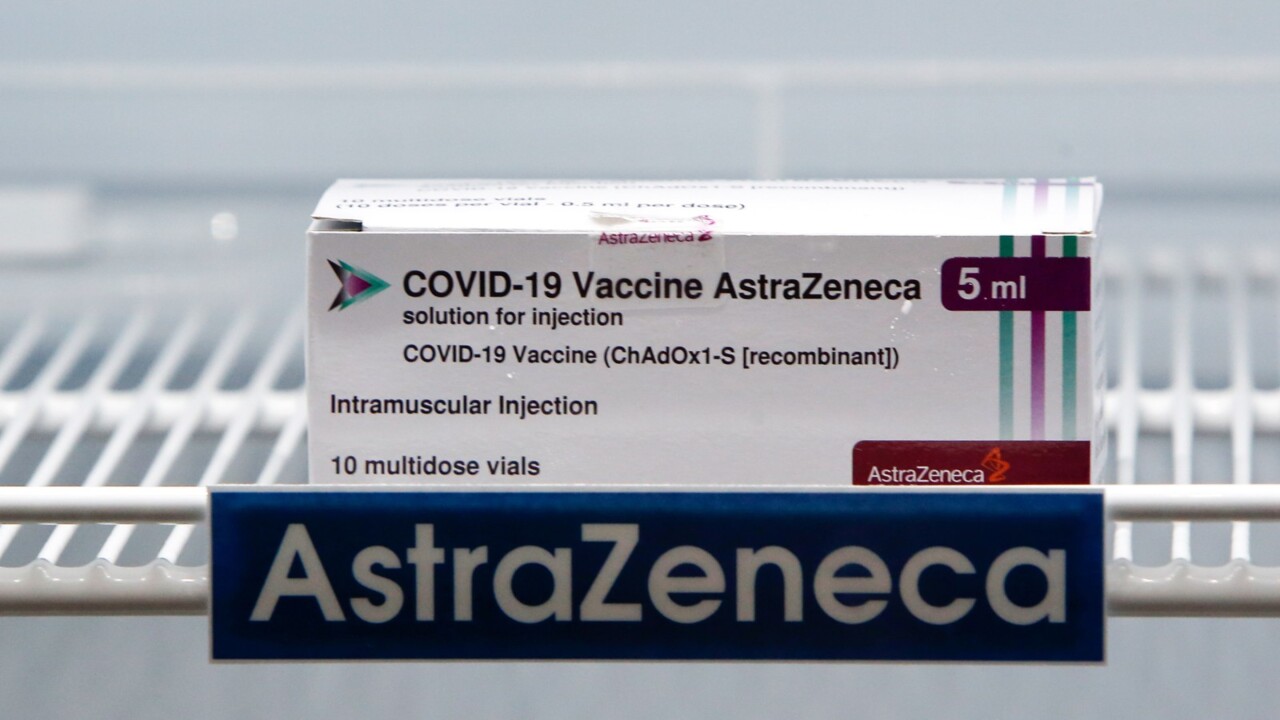
However, the EMA’s report noted it may be associated with clots linked to thromocytopenia, a rare but serious condition involving low levels of blood platelets.
The Australian Technical Advisory Group said there would be no change to the clinical guidance on the use of the AstraZeneca vaccine as a result of the EMA’s findings.
In a statement they said: “ATAGI considers the benefits of vaccination in protecting people in Australia from COVID-19 outweigh the rare potential risk of these rare blood clotting events, and supports the continued rollout of the AstraZeneca vaccine in Australia.”




