Covid world: UN slams ‘stupid’ vaccine rollout
The UN’s chief has lashed the “immoral” wealthy nations that are “hogging” all the Covid vaccines to themselves.
World
Don't miss out on the headlines from World. Followed categories will be added to My News.
UN chief Antonio Guterres has branded rich nations’ vaccine hogging as immoral and stupid, saying choking off poorer countries’ access to jabs risked undermining their own defences against the pandemic.
The World Health Organisation said every country that had fully immunised more than 40 percent of its population was doing too little to stop the Covid-19 crisis, as they should be ensuring doses go to nations left wide open to the virus.
The WHO wanted 10 per cent of the population in every country fully jabbed by the end of September -- a target missed in 56 countries, “through no fault of their own”, said the organisation’s chief Tedros Adhanom Ghebreyesus.

The UN health agency said 1.5 billion vaccine doses were now being manufactured every month -- with less than one week’s production needed to reach the 10 percent target.
“Vaccine inequality is aiding and abetting the Covid-19 pandemic,” Mr Guterres told a virtual press conference alongside Tedros.
“It is allowing variants to develop and run wild, condemning the world to millions more deaths, and prolonging an economic slowdown that could cost trillions of dollars.”
Mr Guterres said that if the virus spread in areas with few vaccinated people, the greater the chances of a variant emerging that could become resistant to vaccines.
“All the vaccination effort made in developed countries to vaccinate the whole of the population, one, two or three times -- all that effort will fall apart. And these people will not be protected.
“So not to have equitable distribution of vaccines is not only a question of being immoral: it is also a question of being stupid.”

Tedros said more than 6.4 billion vaccine doses had now been administered, and almost a third of the world’s population was fully vaccinated against Covid-19.
However, “those numbers mask a horrifying inequity,” he said. “We stand on the precipice of failure” if vaccines are not immediately made widely available, the director-general said.
Tedros said there were enough doses being produced to achieve the WHO’s next target of seeing 40 percent in every country vaccinated by the end of the year -- provided they are distributed equitably.
Meanwhile Tedros also announced that the WHO had now finalised a clinical case definition for post-Covid-19 condition, often called Long Covid.
The definition states that symptoms appear within three months of infection, last for at least two months and cannot be explained by an alternative diagnosis.
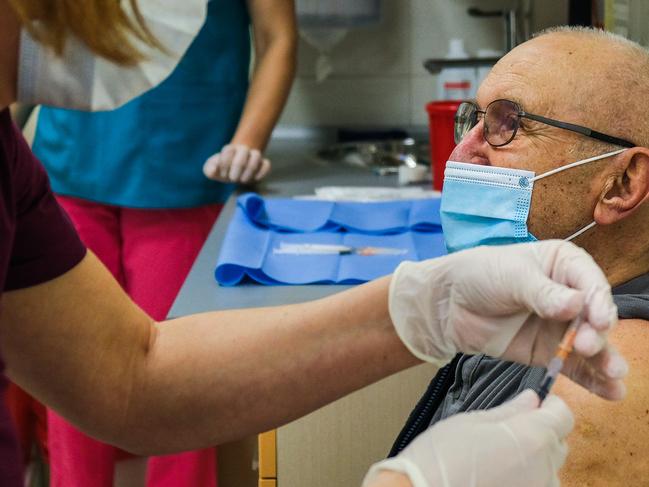
Common symptoms include fatigue, shortness of breath and cognitive dysfunction. They may appear following recovery from acute-stage Covid-19, or persist from the initial illness. Symptoms may also fluctuate over time.
“This standardised definition will help clinicians to identify patients more easily and provide them the appropriate care, and is crucial for advancing recognition and research,” Tedros said.
FINLAND HALTS MORDERNA USE IN YOUNG MEN
Finnish health authorities have said they will stop giving Moderna’s Covid-19 jab to young men, following the example of Nordic neighbours, over fears of heart inflammation side effects.
The announcement comes after neighbouring Sweden, Norway and Denmark announced similar adjustments to vaccine policies on Wednesday.
The countries cited reports of an increased risk of inflammation of the heart muscle (myocarditis) and inflammation of the pericardium (pericarditis), especially for young men and in the weeks following the second dose.
“A Nordic study involving Finland, Sweden, Norway and Denmark has found that men who have received the Moderna Spikevax, men under 30 years of age, have a slightly higher risk than others of developing myocarditis,” Mika Salminen, director of the Finnish Institute for Health and Welfare (THL) told a press conference on Thursday (local time).
Mr Salminen added that “most of these myocarditis cases are mild and transient, healing by themselves with a few days of follow-up, but there is a risk”.
Noting that other vaccines were available, the THL recommended that, as a precautionary measure, Moderna’s jab “should not be given to men and boys under 30 years of age for the time being.”
Like in its Nordic neighbours, THL said that young men were instead recommended for the rival Pfizer/BioNTech jab.
The Swedish Medical Products Agency said in a statement Wednesday that the analysis of the side effects were preliminary and that analysis was “ongoing.”
While Finland has stopped giving the Moderna Covid-19 vaccine to young men, Sweden, Norway and Denmark have paused its use for all young adults and children.
The European Medicines Agency (EMA) authorised the emergency use of Moderna’s Covid-19 vaccine for teens in July.
PFIZER SEEKS US AUTHORIZATION OF COVID VACCINE FOR KIDS
Pfizer has asked for emergency approval from the US Food and Drug Administration for its Covid-19 vaccine for children ages five to 11, the company has announced,
“With new cases in children in the US continuing to be at a high level, this submission is an important step in our ongoing effort against Covid-19,” the pharmaceutical giant wrote on Twitter on Thursday (local time).
“We’re committed to working with the FDA with the ultimate goal of helping protect children against this serious public health threat.”
According to the New York Post, the agency has scheduled its panel of advisers to meet and discuss the application on October 26, making it possible that kids could receive the shot shortly after.
Pfizer has said its vaccine, which was created with German partner BioNTech, was proved to be safe and effective in clinical trials with kids ages 5 to 11.
The vaccine is already authorised for emergency use in those ages 12 to 15 and is fully approved for anyone over the age of 16.
Children have been infected in greater numbers in the latest coronavirus wave driven by the Delta variant, and inoculating young people is seen as key to keeping schools open and helping end the pandemic.
In late September, Pfizer and BioNTech, which co-developed the vaccine, began submitting data to the Food and Drug Administration regulators for the highly anticipated authorisation.
Pfizer tweeted early on Thursday that the two companies had “officially submitted our request” to the FDA “for Emergency Use Authorisation (EUA) of our #COVID19 vaccine in children 5 to 11.
‘REALLY IMPORTANT STEP’
Following the announcement, White House Covid co-ordinator Jeff Zients told CNN that “I think we can all agree that getting a safe and effective vaccine for kids five to 11 is a really important next step in our fight against the virus.”
The FDA has previously said that once the formal submission was completed, the agency would complete its review “likely in a matter of weeks rather than months.” Children in the 5-11 age group received a two-dose regimen of 10 micrograms in the trial, compared with 30 micrograms for older age groups. The shots were given 21 days apart.
The Pfizer-BioNTech vaccine has been granted full FDA approval for those age 16 and up, and the FDA in May authorised its emergency use in children age 12 to 15.
Experts say children are essential to vaccinate to help achieve population immunity against the disease.
Nearly 5.9 million children have tested positive for Covid-19 as of September 30, according to the American Academy of paediatrics.
Vaccines, masks and other mitigation measures against Covid-19 have become deeply political issues in the United States — especially for children.
Early last month the Los Angeles school district, the second biggest in the country, voted that children age 12 and over who attend public schools will have to be fully vaccinated against Covid-19 by the start of next year, in the first such requirement by a major education board in the United States.

The Pfizer vaccine received full, formal approval in the US in August and is therefore technically available to children in younger age groups if prescribed by a doctor, but US authorities have cautioned against doing this until the safety data was in.
Pfizer and BioNTech are also trialling their vaccine on infants aged six months to two years, and on children aged two to five.
Initial data for these groups may come before the end of the year.
COVID JABS ARRIVE IN ANTARCTICA
A delivery of Covid vaccines has arrived in Antarctica to inoculate British researchers stationed in the polar wilderness, the UK foreign ministry said Thursday.
The shipment was delivered to the British Antarctic Survey’s Rothera Research Station, making the outpost the furthest destination in the British Overseas Territories to be supplied with jabs.
The AstraZeneca shots were flown from a Royal Air Force base in Britain via Senegal and the Falklands Islands before reaching the 23-member staff at the facility in the British Antarctic Territory.
The 16,000-kilometre trip across four continents concluded the UK government’s commitment to supply vaccines to all the inhabited UK Overseas Territories and was achieved in partnership with the international development organisation, Crown Agents.

Crown Agents’ chief executive Fergus Drake said the organisation had supplied the vaccines to “literally the ends of the earth”, including the Pitcairn Islands in the Pacific and Tristan da Cunha in the south Atlantic.
Throughout the journey to the planet’s southernmost extremity, vaccines were transported at controlled temperatures to keep the doses at their required two to eight degrees Celsius.
The entire journey had to be carefully planned and executed to ensure the trip was completed in under 92 hours, to avoid spoilage of the vaccines.
Double doses of the vaccines will be available to the 23 members of the “overwintering” team at Rothera, which includes land and marine biologists, meteorologists, engineers, a dive officer, doctor, and chef.
British regulators approved the Pfizer-BioNTech and AstraZeneca vaccines in December last year, and the government began rolling out a mass vaccination program soon afterwards.
Since then, 82.5 per cent of people aged 16 and over have received two jabs of a vaccine, according to government data.

‘NOT OUT OF THE WOODS’
The World Health Organisation’s Covid chief has issued a stark warning to the world: “We’re not out of the woods” in the fight against the pandemic, even if many people thought it was nearly over.
Maria Van Kerkhove, the technical lead for WHO’s Covid-19, said last week 3.1 million known new cases were reported to the UN health agency, and 54,000 more deaths — though the true numbers would be much higher.
“The situation is still incredibly dynamic. And it’s dynamic because we don’t have control over this virus,” she said during a live presentation on the WHO’s social media channels.

“We’re not out of the woods. We’re very much in the middle of this pandemic. But where in the middle … we’re not quite sure yet, because frankly we’re not using the tools we have right now to get us closer to the end.”
She added: “What I really struggle with is in some cities we see ICUs (intensive care units) and hospitals full and people dying — yet on the streets people are acting like it’s completely over.
“You can’t have it both ways.”
Because of the way the world had handled the crisis, Covid-19 would not be eradicated and was here to stay, she added.
Meanwhile, in a surprising statistic, England’s Premier League admits that only seven of its 20 clubs have squads that are more than 50 per cent fully vaccinated even though they are now playing in front of full stadiums.
POLAND CASES RISE
Poland’s government says a fourth wave of Covid infections is gathering pace – with cases rising by about 70 per cent in the past week.
There were 2085 cases reported on Wednesday.
Reuters reports that Poland’s health service was stretched to its limits in the spring by a third wave of the pandemic, during which daily cases exceeded 35,000, but authorities believe vaccinations will help control the number of infections this autumn.
“Today’s data is a very fast flashing red light,” Waldemar Kraska, a deputy health minister, told public broadcaster Polskie Radio 1.
“This is the last moment when we should get vaccinated, because the fourth wave is definitely accelerating, and in those regions where the number of vaccinated people is the lowest.”
Many people in rural southern and eastern regions have decided not to get vaccinated in recent months.
However, Kraska said the government was not planning to return to the large-scale restrictions on public life seen during previous waves of the pandemic.
“We do not plan any restrictions on the economy, if we do, we will pinpoint them, on the level of counties, towns,” he said.
EU COULD START REVIEW OF COVID PILL ‘IN DAYS’
The EU’s medicines watchdog could within days start a review of an oral Covid drug produced by the US pharmaceutical firm Merck, a senior official said on Tuesday local time.
Merck said last week that its drug molnupiravir was shown to reduce hospitalisations by 50 per cent, bringing closer the dream of a simple pill to treat the coronavirus pandemic.
“What I can say is that indeed we will be considering whether to start a rolling review for this compound in the next days,” Marco Cavaleri, head of vaccine strategy at the European Medicines Agency, told journalists.
Cavaleri said the Amsterdam-based agency was aware of some “top-line results that are being communicated by the company” about its new drug.

Merck said on Friday it planned to submit an application to the US FDA and other regulatory bodies worldwide for the drug.
It said trial results on 770 patients showed there were no deaths among patients who received the drug compared to eight deaths in the placebo group.
Under a rolling review, the EMA can speed up drug approvals by examining safety and efficacy data as they are released, instead of waiting until after a formal application for authorisation is filed by the manufacturer.

Approval can take several months.
The Merck pill has been hailed as a possible breakthrough because until now Covid therapeutics — which treat the disease as opposed to vaccines, which prevent it — have so far been administered intravenously.
The EMA meanwhile raised hopes that vaccine booster doses could offer a long period of protection against Covid.
The agency on Monday authorised a third booster dose of the Pfizer/BioNTech vaccine for people aged 18 and over, six months after their initial two jabs, amid fears that it offers diminishing protection.
“What we’re seeing at least with this vaccine is that the immune response is indeed much higher than what we’ve seen after the second dose,” Cavaleri said.
“Which means that potentially we will have quite remarkable amounts of neutralising antibodies, also for much longer than six months.”


