Jocelyn Suiter: A simple label on medication could protect our children
PARENTS of asthmatic children must be made fully aware of medication’s potential side effects. It isn’t enough to rely on Dr Google, writes Jocelyn Suiter.
Opinion
Don't miss out on the headlines from Opinion. Followed categories will be added to My News.
‘MUMMY, I can’t sleep ... Mummy, I really can’t sleep.” It’s almost midnight. My son is standing next to my bed, shouting in my ear. Again. No amount of back rubs, essential oils, glasses of milk, threats, punishments or rewards can help.
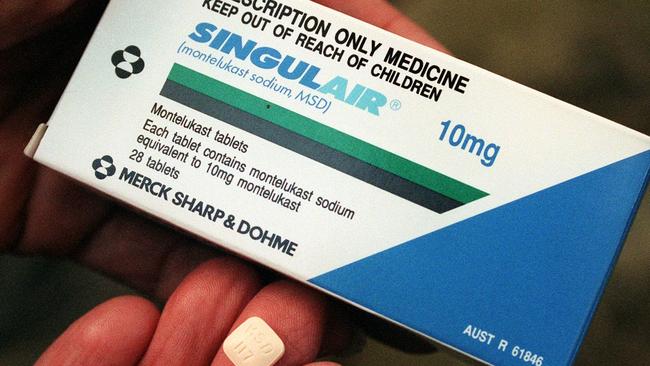
For more than 12 months, my nine-year-old son didn’t sleep for more than a few hours a night. And it almost tore our family apart. What we didn’t know was that he was suffering side-effects from an asthma drug, Singulair (the generic name for which is montelukast).
He began taking the tablets every day after having a life-threatening allergic reaction to a walnut. A specialist recommended Singulair to control his asthma and to help with the response to his allergies. Neither the specialist nor our GPs warned us about any side-effects. In fact, when I asked the question, one even said there were none. There was no information leaflet inside the box and no warnings on the packaging.
The insomnia began slowly, then came the anxiety. We thought it was as a result of his near-death anaphylaxis experience. Then came the daily tantrums, the tears and screaming. We thought it was related to his lack of sleep.
The family would tiptoe around him, trying not to spark an emotional outburst. It’s difficult to describe the difficulty of living with a small child who is on the edge, but here’s a snapshot: we were on a family holiday to Disneyland, “the happiest place on Earth”, and my son was lying on the ground, screaming.
We were at the point of sending him to be assessed by a psychiatrist when a light bulb went off in my head. An internet search listed the side-effects of Singulair — and my son had most of them.
So who is responsible for warning about the side-effects of medication? The manufacturer? The prescribing doctor? The dispensing pharmacist? Or, in this digital age, is it somehow accepted that the patient has to be Dr Google and do the research themselves?
One Melbourne mother, Vanessa Sellick, an advocate for a global Facebook group of about 2500 affected parents, is leading the push for the Therapeutic Goods Administration to mandate warning labels on the packaging of Singulair.
The manufacturer, Merck, maintains that tens of millions of people worldwide use Singulair safely and it is up to doctors to warn patients of any side-effects. It says the side-effects are outlined in product information that is available online. In 2013, the TGA published warnings of potential neuropsychiatric side-effects, but it appears many doctors are not passing that warning on to their patients.
The Australian Medical Association chair of general practice, Dr Richard Kidd, has defended GPs. He says the chance of side-effects was too low to warrant unnecessarily frightening parents. “The consumer medication pamphlet is supposed to be given in the box when the script is filled,” he told ABC Radio several months ago.

But the Pharmacy Guild responded by saying that it was up to the doctors to adequately warn patients of side-effects: pharmacists are not required to provide the Consumer Medicines Information.
The true incidence of side-effects to Singulair is unknown; there is a lack of research on the long-term use of the drug and why it affects some children.
Like our family, many parents do not link their children’s neuropsychiatric struggles — including depression, anxiety and suicidal thoughts — with an asthma drug.
THERE is no doubt the medication is effective for most patients. After Melbourne’s tragic thunderstorm asthma event, in which eight people died in late November, it is likely many more children — and adults — have been prescribed Singulair.
Our group is not asking that it be taken off the shelf. We are asking for a fluorescent warning label that outlines potential neuropsychiatric side-effects to be placed on the packaging.
In the United States, after a teenager committed suicide while taking montelukast, warning labels on packaging were mandated. They say: “Call doctor if you experience mood changes, sadness, depression or fear.”
But the TGA and a representative for federal Health Minister Sussan Ley have said such warning labels are not necessary in Australia, adding that all medicines could have unwanted side-effects and the potential risks should be discussed with medical practitioners.
A warning label on the box could have alerted my family to the risks and prevented my son’s trauma.
The day after he stopped taking Singulair, he slept through the night for the first time in a year.
We have warning labels for children on pyjamas and plastic bags — so why not on this medication?
Jocelyn Suiter is a Herald Sun journalist


