‘It actually works’: Patients develop antibodies in COVID-19 vaccine breakthrough
Researchers have had a major win in the fight against the coronavirus after patients trialling a COVID-19 vaccine developed antibodies that could protect them from the virus.
News
Don't miss out on the headlines from News. Followed categories will be added to My News.
In a major breakthrough patients trialling a COVID-19 vaccine have developed antibodies that could protect them from the virus.
Moderna, the US company behind the jab, was the first company to begin human trials of a coronavirus vaccine when it injected around 45 people with the experimental product in March.
The firm’s chief medical officer Dr Tal Zaks said overnight the vaccine “actually works” due to its ability to stimulate the human immune system.
The company has reported that within two weeks of receiving the vaccine everyone of the volunteers developed antibodies that may protect them from falling sick with COVID-19.
Patients in the trial were given varying doses of the vaccine and those on the lowest dose produced antibodies at similar levels to people who naturally caught the coronavirus.
Those who received a medium dose developed more antibodies than naturally recovered patients, the company reported.

Some of those given the highest dose of the vaccine exhibited “systemic symptoms” after the second dose of the vaccine but these were transient the company said.
“These interim Phase 1 data, while early, demonstrate that vaccination with mRNA-1273 elicits an immune response of the magnitude caused by natural infection,” Dr Zaks stated in the firm’s media statement.
There were minor side effects such as soreness at the injection site but the vaccine was generally safe, the company said.
“mRNA-1273 was generally safe and well tolerated, with a safety profile consistent with that seen in prior Moderna infectious disease vaccine clinical studies,” the company said.
The research has not been published or peer reviewed and tested whether it was safe to use the vaccine in humans.
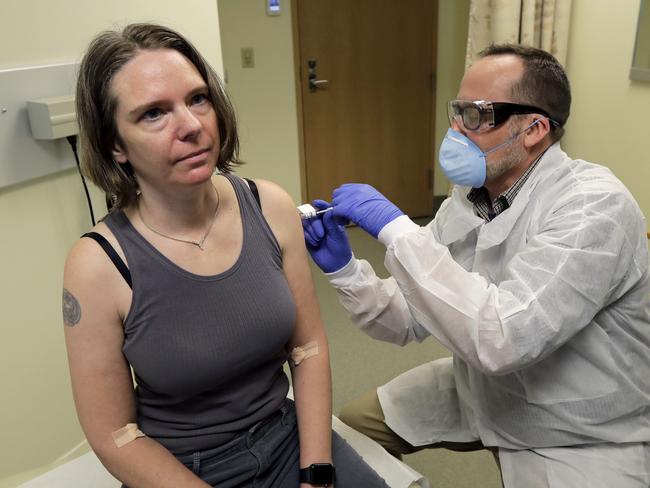
The company is planning the next, larger phase of clinical testing with 600 volunteers to be given the vaccine in July.
Dr Zaks predicts the vaccine is likely to go on the US market at “about the end of the year, the start of next year”.
The tests have not yet proven that the vaccine prevents people getting coronavirus.
WARNING AGAINST COVID-19 ‘VACCINE ‘NATIONALISM
Former health department secretary Jane Halton has issued a warning about vaccine nationalism which could threaten fast access to a COVID-19 vaccine worldwide.
Countries or pharmaceutical companies that tried to monopolise access to the first successful vaccine would not be helping themselves or the eradication of the virus, she said.
“We do need to understand if this virus is everywhere in the world and vulnerable people are not protected, everyone is vulnerable,” she said.
Ms Halton who is the chair of the Centre for Epidemic Preparedness Innovation which is funding the development of multiple COVID-20 vaccines said first responders in the health profession should get the first access to the vaccine followed by the elderly and those with chronic illnesses because they were most vulnerable to the virus.
It could take some time to produce the 8 billion doses of the vaccine needed to cover the world’s entire population and it might be that two doses of the vaccine was needed to produce full immunity, she said.

VACCINE ‘READY BY SEPTEMBER’
That call comes as a coronavirus vaccine could be available as soon as September as researchers in the UK race to be the first to crack the code that stops the deadly disease.
Britons will get first access to a coronavirus vaccine being developed by Oxford University with pharmaceutical giant AstraZeneca poised to make 30 million for the UK – if it works.
Business Secretary Alok Sharma announced a deal has been done between the university and the company to manufacture the vaccine which is currently in clinical trials.
He pledged an additional $A160 million to accelerate the development of the vaccine – on top of a previous $A90 million pot of cash – so that mass production can start as soon as possible if it is proved to be effective.
Mr Sharma said the Oxford project is “progressing well” and that another vaccine effort by Imperial College London is “also making good progress”.
However, he cautioned that despite the growing optimism there are “no certainties” and there may never be a vaccine developed capable of tackling the deadly disease.
Mr Sharma also revealed that six drugs designed to treat coronavirus have now entered initial live clinical trials.
The world is yet to identify a drug clinically proven to treat the disease.
DON’T GET YOUR VACCINE HOPES UP, SAY EUROPEAN LEADERS
Don’t pin your hopes on a vaccine being able to provide the world with a return to normal life.
That’s the message issued by Italian Prime Minister Giuseppe Conte and British Prime Minister Boris Johnson.
The dire comments came as governments worldwide and many US states struggled with restarting economies blindsided by the pandemic.
With 36 million newly unemployed in the US alone, economic pressures are building even as authorities acknowledge that reopening risks setting off new waves of infections and deaths.
Pushed hard by Italy’s regional leaders and weeks in advance of an earlier timetable, Mr Conte is allowing restaurants, bars and beach facilities to open Monday, the same day that church services can resume and shops reopen.
“We are facing a calculated risk, in the awareness … that the epidemiological curve could go back up,” Mr Conte said. “We are confronting this risk, and we need to accept it, otherwise we would never be able to relaunch.” He added that Italy could “not afford” to wait until a vaccine was developed.


Health experts say the world could be months, if not years, away from having a vaccine available to everyone despite the scientific gold rush now on to create one.
Britain’s PM Boris Johnson, who was hospitalised last month with a serious bout of COVID-19, speculated on Sunday that a vaccine may not be developed at all, despite the huge global effort to produce one.
“I said we would throw everything we could at finding a vaccine,” Mr Johnson wrote in the Mail on Sunday newspaper. “There remains a very long way to go, and I must be frank that a vaccine might not come to fruition.”
Coronavirus has infected over 4.6 million people and killed more than 312,000 worldwide, according to a tally by Johns Hopkins University that experts say under counts the true toll of the pandemic.
US APPROVES HOME TESTING KITS
The US Food and Drug Administration has authorised an at-home sample collection kit that can then be sent to specified laboratories for COVID-19 diagnostic testing.
The Everlywell emergency use authorisation (EUA) kit is authorised to be used by individuals at home who have been screened using an online questionnaire that is reviewed by a healthcare provider.
This allows an individual to self-collect a nasal sample at home using the kit.
The FDA has also authorised two COVID-19 diagnostic tests performed at specific laboratories for use with samples collected using the home testing kit.
“The authorisation of a COVID-19 at-home collection kit that can be used with multiple tests at multiple labs not only provides increased patient access to tests, but also protects others from potential exposure,” said Dr Jeffrey Shuren, director of the FDA’s Center for Devices and Radiological Health.
DOGS TRAINED TO DETECT COVID-19 BEFORE SYMPTOMS APPEAR
New trials are underway in the UK to see if specially trained “COVID dogs” might be able to detect coronavirus in humans even if they are asymptomatic, or before symptoms occur.
Medical Detection Dogs have already been successfully trained to detect the odour of many different diseases in humans, such as cancer, malaria and Parkinson’s disease.
The first phase of the trial will be carried out by the London School of Hygiene and Tropical Medicine (LSHTM) in collaboration with the charity Medical Detection Dogs and Durham University, backed by £500,000 ($A944,000) of government funding.
Scientists are hopeful that the dogs, a mixture of labradors and cocker spaniels, can be trained to detect coronavirus in humans from odour samples, even if they are not showing symptoms.
“Bio-detection dogs already detect specific cancers and we believe this innovation might provide speedy results as part of our wider testing strategy,” said Lord Bethell, Minister Innovation.
“Accuracy is essential so this trial will tell us whether ‘COVID dogs’ can reliably detect the virus and stop it spreading.”
If successful, these dogs could provide a fast and non-invasive detection method.
It is one of a number of testing measures being explored in order to ensure the government’s response to the virus is as extensive as possible.
During the initial phase of the trial, NHS staff in London will collect odour samples from people who are infected with coronavirus and those who are uninfected. The six bio detection dogs will then be thoroughly trained to identify the virus from the samples.
The dogs have been proven to be able to screen up to 250 people per hour, and can be trained to detect the odour of disease at the equivalent dilution of one teaspoon of sugar in two Olympic-sized swimming pools.
Professor James Logan, lead researcher and Head of the Department of Disease Control at the London School of Hygiene and Tropical Medicine, said, “Our previous work has shown that malaria has a distinctive odour, and with Medical Detection Dogs, we successfully trained dogs to accurately detect malaria. This, combined with the knowledge that respiratory disease can change body odour, makes us hopeful that the dogs can also detect COVID-19.”

VACCINE MONKEY TRIALS YIELD PROMISING RESULTS
A vaccine trial in the US appears to have prevented six rhesus macaque monkeys from developing COVID-19.
The vaccine is now undergoing clinical trials in humans, however it is uncertain if the vaccine will offer the same immunity to humans.
The US trial involved researchers from Oxford University and the US government’s National Institute of Health (NIH) and saw a group of monkeys exposed to the SARS-Co-V-2 virus.
The six monkeys who received the vaccine had less of the virus in their lungs and airways, and the vaccine also appeared to protect the animals against developing pneumonia.
There was no development of “immune-enhanced disease” in the monkeys, either, where a vaccine can trigger a worse response to a disease.
In the past, the development of immune-enhanced disease has occurred in trials for a SARS vaccine.
The immune systems of rhesus macaque monkeys are similar to those of humans.
Professor Stephen Evans at the London School of Hygiene and Tropical Medicine described the monkey trial results as “high quality” and “very encouraging”.
A visiting professor in pharmaceutical medicine at King’s College London, Dr. Penny Ward described the vaccine results as “helpful”.
The vaccine is made from a small part from the virus, designed to train the body to recognise a unique part of the virus and know what it needs to do to fight the virus when presented with it in its entirety.
The vaccinated monkeys produced antibodies capable of fighting the virus.
UK trials on more than 1000 human volunteers are currently underway at the University of Oxford.
Meanwhile, trials in the UK on more than 1,000 human volunteers are currently taking place through the University of Oxford.
There are more than 100 experimental coronavirus vaccines currently being developed.
US SLAMS CHINA FOR ‘HACKING’ VIRUS RESEARCH
The US has slammed the Chinese government for attempting to hack its research into a coronavirus vaccine.
Secretary of State Mike Pompeo called on Beijing to “cease this malicious activity”.
“The United States condemns attempts by cyber actors and non-traditional collectors affiliated with the People’s Republic of China (PRC) to steal US intellectual property and data related to COVID-19 research,” Mr Pompeo said in a statement released Thursday, local time.
The FBI and Department of Homeland Security on Wednesday warned of the cyber espionage attempts on US scientists.
“The potential theft of this information jeopardises the delivery of secure, effective, and efficient treatment options,” the statement from the FBI said.
Mr Pompeo said China’s “behaviour in cyberspace is an extension of its counter-productive actions throughout the COVID-19 pandemic”.
“While the United States and our allies and partners are co-ordinating a collective, transparent response to save lives, the PRC continues to silence scientists, journalists, and citizens, and to spread disinformation, which has exacerbated the dangers of this health crisis,” Mr Pompeo said.

The cyber warning comes after China has been accused of covering-up the origin of the coronavirus and delaying warning the world it was contagious so they could stockpile medical supplies.
Australia and the US are among countries to call for an independent investigation into how COVID-19 spread from Wuhan to the world, infecting more than 4.3 million and killing 300,000 people.
Beijing on Thursday denied responsibility for the cyber attacks.
“China expresses strong dissatisfaction and firm opposition to such smearing,” said foreign ministry spokesman Zhao Lijian.
“Judging from past records, the US has carried out the largest cybertheft operations worldwide.”
The coronavirus has plunged relations between Washington and China to new lows and saw US President Donald Trump hint this week at withdrawing from a recent trade deal.
“We got a lot of things going with China. We’re not happy about China — I will tell you that,” Mr Trump said Thursday morning local time at the White House.
“The ink wasn’t dry on a great trade deal, and all of a sudden, the plague comes in from China. We’re not happy about it.”
MACRON MEETS WITH VACCINE PRODUCERS
French President Emmanuel Macron’s top officials will meet with executives of pharmaceutical giant Sanofi early next week, his office said Thursday, after the firm’s CEO said the United States would be the first recipient of a COVID-19 vaccine it is racing to develop.


“This vaccine must be a global public good, which is not submitted to market forces,” the presidency said in a statement.
It comes as Russian President Vladimir Putin has met in the Kremlin with top Russian officials about genetic research and plans for Russia to launch three top research centres in the field of a coronavirus vaccine.
Rinat Maksyutov, the head of the Vektor State Virology and Biotechnology Centre, told Mr Putin at the meeting that research was ongoing on several potential vaccines against the coronavirus.

MOSCOW LAUNCHES IMMUNITY TESTS
The top secret lab complex in Siberia has developed six prototype vaccines and Mr Maksyutov told Mr Putin that “three promising ones” had been chosen for the next stage of preclinical studies.
He said he hoped to be able to register an experimental coronavirus vaccine in September.
“I am very much counting on your work being finished … and the registration of this vaccine against coronavirus being carried out in September,” Mr Putin told Mr Maksyutov.
Mr Putin said the research centre needed to ensure its intellectual property rights on the vaccine.
It was unclear if he meant that a vaccine should be ready for use by September. The Vektor laboratory conducted secret biological weapons research in the Soviet era and stockpiles viruses ranging from ebola to smallpox.
MORE NEWS:
Harry and William ‘back in touch’ in royal reconciliation
Royal family pays tribute to Aussie nurses in the front line
Kim ‘missing again’ for almost two weeks raising new doubts
Jobs: 600,000 lost in April with PM focused on reopening economy

U.S. HEADED FOR ‘DARKEST WINTER’ IN MODERN HISTORY
Meanwhile, in Washington, Dr. Rick Bright, a top U.S. immunologist who says he lost his government job because he warned the Trump administration to prepare for the coronavirus pandemic, told Congress the U.S. lacks a plan to produce and fairly distribute a coronavirus vaccine when it becomes available.
“Our window of opportunity is closing,” Dr. Bright said in his prepared testimony. “If we fail to develop a national co-ordinated response, based in science, I fear the pandemic will get far worse and be prolonged, causing unprecedented illness and fatalities.”
Whistleblower Dr. Bright warned the U.S. lacks a plan to produce and fairly distribute a coronavirus vaccine when it becomes available.
Dr. Bright told a congressional panel the nation could face “the darkest winter in modern history” unless leaders act decisively.
