Medtronic bone graft device no longer available for spinal surgeries in Australia
A controversial product used in 40,000 spinal surgery patients has been pulled weeks after surgeons were caught inserting it in unapproved ways. But the move has left doctors divided.
Health
Don't miss out on the headlines from Health. Followed categories will be added to My News.
A controversial bone graft product used in 40,000 spinal surgery patients has been pulled from the Australian market weeks after surgeons were caught inserting it in unapproved ways.
It comes as a News Corp investigation has uncovered a separate pain device implanted into patients’ spines left one a quadriplegic and another hospitalised for six months and near death.
Many other patients said they were barely able to sit or walk after spinal surgery involving implants and bone growth products yet these adverse events had not been reported to the nation’s medical regulator.

Spinal fusion surgery for uncomplicated back pain costs taxpayers and health fund members hundreds of millions of dollars a year. It drives up health fund premiums and an expert committee found no evidence it works.
In many cases patients are left worse off, with some requiring up to six surgeries to manage their pain.
News Corp revealed in February spine surgeons were using a product that stimulates bone growth — Medtronic Infuse Bone Graft — in unapproved ways without the cage meant to contain it.

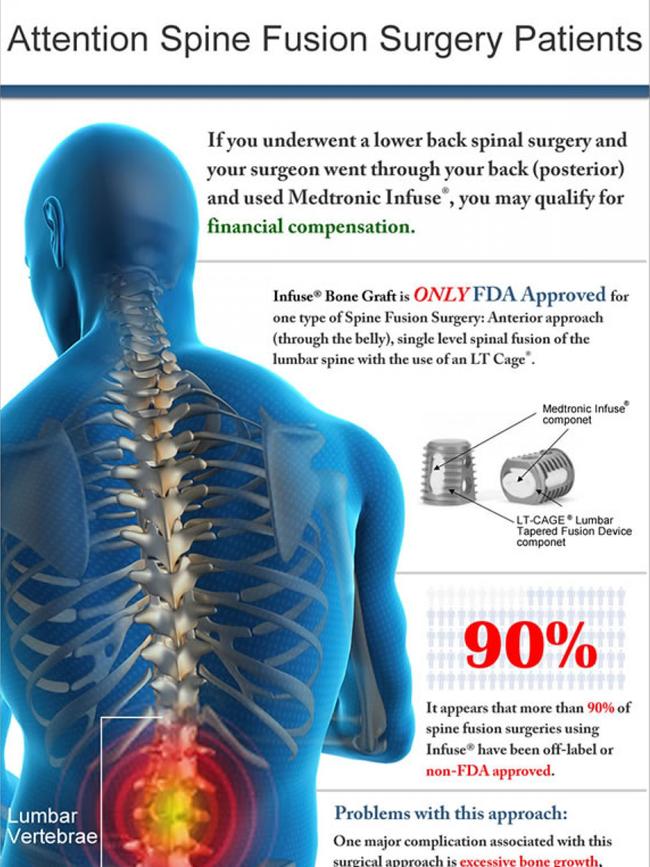
Just weeks later Medtronic removed the product from sale in Australia and it can now only be supplied under a special access scheme.
The product was the subject of 10,000 lawsuits in the US, which cost Medtronic $460 million in settlements, after it was connected to adverse outcomes including death, cancer, inflammation in nearby tissues and bone and urinary problems.
News Corp can also reveal a senior spinal surgeon was referred to a drug diversion program after being caught with cocaine in his possession, while another had conditions placed on his medical practice after failing to report adverse events in patients.
Many spinal surgeons have taken payments and luxury overseas trips to European ski resorts, the Bahamas and Los Vegas funded by medical device companies that make the contentious products.
University NSW Professor Ian Harris said he does not do spinal fusion surgery any more because the evidence it works for back pain is not there.
“I don’t think it should be done at all,” he said.
Workers compensation data shows there is an 18 per cent chance of a second surgery within two years.
Just three per cent of patients return to pre-fusion duties at work and 80 per cent are still getting physio or opioids two years after their procedures.
Professor Vagg said his college had been trying for seven years to set up a medical device registry for implanted pain control devices to monitor the outcomes in patients.
“We have particular concerns about spinal cord stimulation devices,” he said.
“Certainly I’m aware of at least one very serious case where the outcome of a neuromodulation device procedure was quadriplegia,” he told News Corp.
Private Healthcare Australia chief Dr Rachel David said health funds had major concerns about the lax regulation of medical devices.
Health fund data showed a staggering one in nine patients who had spinal surgery were being re-admitted to hospital within the same financial year.
Dr David said there were concerns some surgeons were captives of the medical device companies.

Spine Society of Australia president Dr Matthew Scott-Young said the withdrawal of Bone Graft from Australia would leave patients including those suffering from scoliosis and adult spinal deformity worse off.
He said surgeons would now have to harvest patients’ own bone for the procedures.
“As a result, our patients will experience more post-operative pain, longer
operative times, more blood loss, increased hospital stays, more adverse events, and more
frequent readmissions to hospital,” he said.
Mary Lynne Cochrane has had 23 surgeries including three spinal surgeries trying to treat the rheumatoid arthritis she developed in her twenties.
“If I knew what I know now I wouldn’t have had them,” she said.
A member of the religious order the Sisters of good Samaritans she has difficulty walking any distance and for a time became addicted to opioids as she tried to control her pain.
There are days when she wakes up and is unable to move and she suffers nerve pain from scar tissue “that is worse than any pain I’ve had”.

Mother-of-three Tracie Jarvis ruptured a disc in her back in 2006 lifting heavy loads in her family run pizza restaurants.
Her first surgery failed to fix the problem and her second surgery — where a prosthetic disc was implanted in her spine — could not dull lingering nerve damage, which left her in agony.
In 2008 she agreed to have a neuromodulation device implanted to control the pain but the surgery gave her with septicaemia, a golden staph infection and abscesses on her spinal cord and her heart.
“I was in hospital for six months, I wasn’t meant to survive,” the 50-year-old said.
The neuromodulation device had to be removed without a general anaesthetic and she now can’t sit upright for more than 15 minutes, finds it hard to walk and had to give up work.
Years later she had a second neurological device implanted, which made a difference to pain in her legs but not her back. It has since been removed.

The fallout from the surgical disaster left her mostly bedridden and she lost contact with her friends.
“My world just disintegrated,” she said.
Neil Gibbons of Brisbane had suffered back pain for more than 30 years before having fusion surgery which he considers “the worst decision I made in my life”.
The 69-year-old, who used to be in the military, could not move after the surgery which saw rods put into his vertebrae and bone graft product inserted.
“I couldn’t sit up and the pain was worse than prior to the operation,” he said.
He developed new pain in the L5 disc that ran through to his hip and developed numbness in his calf muscle.
“I can walk but I’ve definitely got a limp and the knee tends to collapse and it hurts the hip,” he said.
He has now been diagnosed with cancer “right behind where the spine surgery was”.

Jeremiah Thomas was fit and active university student when he injured his sacroiliac joint shifting a pot belly stove while moving house.
For six weeks the pain was so bad he couldn’t sleep and had to give up his Psychology Degree.
Now aged 46, he can’t work because he is disabled by complex regional pain syndrome.
Mr Thomas who lives in Hobart attended a pain clinic, tried physiotherapy, exercise regimes and Botox injections — none of which helped.
“I went from being able to hike and fly fish to being unable to walk meaningful distances, isolated in bed 15 hours a day,” he told News Corp.
MORE NEWS
The 600 medicines now in short supply
New cancer drug works like a guided missile
Billion dollar plan to make more medicines in Australia
Why energy drinks are so dangerous for teens

He had a neuromodulation device implanted but his burning pain quickly became extreme.
“I had problems wearing clothes, even the touch of clothes was a problem. “
His feet went red and his nail and hair growth slowed as the device affected his autonomic nervous system.
He can’t have the device removed because of the risk of further damage.
“This is just a train wreck of people,” he said.
“My hands are shaking, my life has changed so badly I’ve been treated so badly by the medical community but people just walk away and wash their hands.”
“It’s one person against the medical community.”
Originally published as Medtronic bone graft device no longer available for spinal surgeries in Australia
