Coronavirus: US approves Pfizer’s COVID-19 vaccine
A COVID-19 vaccine is a step closer in the US after a government advisory panel backed Pfizer’s jab, as Ellen tested positive.
Coronavirus
Don't miss out on the headlines from Coronavirus. Followed categories will be added to My News.
A panel of experts advising the US Food and Drug Administration has recommended that the US regulator authorise the use of the Pfizer COVID-19 vaccine.
The FDA is expected to approve its use “within days” and the vaccine is likely to be rolled out almost immediately to nursing home residents and healthcare workers.
The vaccine advisory panel of the FDA, which includes scientists, infectious diseases doctors and statisticians, has voted in favour of emergency authorisation of the vaccine for people aged 16 and over.

Meantime, the Morrison government has cancelled a billion-dollar deal to buy more than 50 million doses of the University of Queensland’s potential coronavirus vaccine.
The decision was made after a number of people who took part in trials falsely tested positive HIV test results.
The University of Queensland, in partnership with Australian global biotech company CSL, is cancelling its current clinical trials following the discovery. It informed the federal government of the development on Monday.
Nine Newspapers reported that sources with knowledge of the current trials said pathology tests had in the past weeks confirmed the positives were in fact false and the health of the participants has not been put at risk.
The National Security Committee of Cabinet agreed to terminate the purchasing agreement on Thursday, following expert health advice and fears the revelation would severely damage the Australian public’s confidence in the COVID-19 vaccination program, which is expected to begin early next year.
A health official, not authorised to speak publicly, told Nine Newspapers the government had moved swiftly in the past 48 hours to secure tens of millions more doses of candidate vaccines, including from Oxford University-AstraZeneca, to cover for the 51 million doses the homegrown product was to supply.
ELLEN HAS COVID-19
Troubled talk show host Ellen DeGeneres has confirmed she tested positive for coronavirus. “Fortunately, I’m feeling fine right now,” the Ellen host tweeted.
“Anyone who has been in close contact with me has been notified, and I am following all proper guidelines. I’ll see you all again after the holidays. Please stay healthy and safe. Love, Ellen.”
It has been a rough year for DeGeneres, who faced allegations of a “toxic” workplace environment and led to the sacking of a string of senior staff members. Since the accusations, her daytime talk show Ellen has reportedly suffered a dip in ratings, sponsors and support from Hollywood stars.

Meanwhile, COVID case numbers in the US are thought to be far higher than the numbers being reported.
America topped 15 million cases of COVID-19 this week, but a top Centres for Disease Control and Prevention official said that number is likely exponentially higher — by as much as seven times.
Aron Hall, co-lead of the agency’s epidemiology task force, offered the startling statistic during a virtual hearing over Pfizer/BioNTech’s coronavirus vaccine.
“The total estimated number of infections is likely two to seven times greater than reported cases,” Hall said, adding that the evidence was based on seroprevalence surveys and models.
“Though less than 10 per cent of population in most states had evidence of previous infection through September.”

This week, America surpassed 15 million cases of coronavirus as infections and hospitalisation continue to soar into what officials are calling the darkest months of the pandemic.
More than 289,000 people have died from the virus.
Dr Hall said the death toll is also likely higher.
“We do feel as with hospitalisation and illnesses that the reported number of deaths is likely an underestimate of the true number of deaths,” he said.
US REGULATORS MEET ON VACCINE APPROVAL
It comes as US regulators met on Thursday (local time) to weigh emergency approval for a coronavirus vaccine a key medical journal deems a “triumph” as the United States logged more than 3000 virus deaths in just one day.
With northern hemisphere countries hit by a pandemic winter surge, Britain this week became the first western country to roll out the Pfizer-BioNTech vaccine.
Canada is preparing to follow suit after also approving it.
EU countries eagerly awaited a green light for vaccines that the bloc’s own watchdog said remained on track for approval despite a two-week-long cyberattack, which is under investigation.

Independent experts convened by the US Food and Drug Administration (FDA) discussed whether to grant emergency approval for Pfizer-BioNTech’s vaccine and allow America to become the next country to move ahead with mass immunisation.
They were due to hold a non-binding vote at the end of the day and the US regulator will determine after that whether to issue an emergency use authorisation.
Momentum was building as the New England Journal of Medicine published full results of a clinical trial of the vaccine, which confirmed it was 95 per cent effective with no serious safety issues.
“The trial results are impressive enough to hold up in any conceivable analysis. This is a triumph,” an accompanying editorial said.

Nevertheless, the two dozen voting members of the Vaccines and Related Biological Products Advisory Committee (VRBPAC) were expected to mull outstanding issues closely in a live streamed event held over the course of the day.
These include difficult questions like when people who were on the placebo-arm of the trial should receive the vaccine, a tricky debate that weighs the need for more controlled data against paying back altruistic volunteers for their service.
The editorial that accompanied the study flagged certain “minor issues”. Questions include whether unexpected safety issues may arise when the number of people vaccinated grows to millions and possibly billions of people.
Also unknown is whether more side effects will emerge with longer follow-up, how long the vaccine remains effective, whether it will limit transmission and how it will work in children, pregnant women, and immunocompromised patients.

The FDA is likely to issue an allergy warning if it approves the vaccine following Britain’s lead after two health care workers there suffered reactions and needed treatment.
The United States is the worst-hit nation in the world, with more than 15 million known infections and close to 290,000 deaths.
It registered more than 3,000 deaths in 24 hours — the highest daily toll since April.
The virus has killed at least 1,570,398 people worldwide since the outbreak emerged in China last December, according to a recent tally.
The US hopes to vaccinate 20 million people this month, with long term care facility residents and health care workers at the front of the line. The goal is to reach 100 million by the end of February and the whole population by June.
TRUMP’S LAWYER LEAVES HOSPITAL
Meanwhile, Donald Trump’s personal lawyer, Rudy Giuliani, has left the hospital after a four-day stay as he underwent treatment for coronavirus.
Mr Giuliani tweeted about his condition Thursday morning (local time) after being discharged from Georgetown University Medical Centre in Washington, DC, on Wednesday night.
The former New York mayor said he was “back 100 [per cent],” and had “lost little time.”
Mr Giuliani thanked the medical staff and touted the care he received.
“My treatment by the nurses and staff at Georgetown Med Star Hospital was miraculous. I walked in with serious symptoms. I walked out better than ever,” he wrote in the second of a pair of tweets, going on to mention the doctors by name.
According to the New York Post, journalists waiting outside the hospital for Mr Giuliani reported that he appeared to be in good spirits when leaving, flashing a thumbs up to them from the passenger’s seat as his car drove away.
Mr Giuliani spoke about his condition in an interview by phone Tuesday on his own US radio show, saying he was “pretty much” symptom-free and “doing fine.”
The chief defender Mr Trump’s widespread voter fraud claims is now heading back to work, testifying Thursday before the Georgia legislature on what he referred to on Twitter as “election theft tapes.”

JURY OUT ON SAFETY OF VACCINE
Australia’s medicines regulator has not yet decided whether to advise people with allergies to avoid the Pfizer COVID-19 vaccine even though UK authorities have done so.
The Therapeutic Goods Administration (TGA) told News Corp “it is too early to speculate which warnings may be included” for the vaccine.
The TGA said it “continues to receive and assess ongoing vaccine efficacy and safety data from Pfizer, and other vaccine developers, as part of the Australian provisional registration assessment process”.
“We are also working closely with international regulators, including in the United Kingdom, to ascertain real-world safety information as it comes to hand. All available safety information will be considered as part of our assessment process,” the Administration said.
Once a vaccine is approved in Australia it will have Product Information and Consumer Medicine Information which will describe potential side effects in addition to special warnings and precautions.

The TGA said when a vaccine is provisionally approved, it will be closely monitored by the TGA’s safety monitoring system so that any potential safety issues are rapidly detected and responded to appropriately.
It comes after two British health workers who were vaccinated on the first day of the rollout of the Pfizer and BioNTech vaccination needed to use an EpiPen after their inoculation.
Both had a history of allergic reactions, which meant they were required to carry an EpiPen with them in case of an anaphylactic shock.
However, despite the issue, the vaccine has still been declared safe and the vaccination program was continuing.
The Medicines and Healthcare products Regulatory Agency (MHRA) issued the warning about allergic reactions on Thursday Australian time.
CNN found a man on the street in London, Martin Kenyon, age 91, who was one of the first people in the world to receive the coronavirus vaccine.
— Liam Stack (@liamstack) December 8, 2020
“Well, there’s no point in dying now when I’ve lived this long, is there?†pic.twitter.com/WajmeKbyoq
“Any person with a history of a significant allergic reaction to a vaccine, medicine or food (such as previous history of anaphylactoid reaction or those who have been advised to carry an adrenaline autoinjector) should not receive the Pfizer/BioNtech vaccine,” the warning said.
“Resuscitation facilities should be available at all times for all vaccinations. Vaccination should only be carried out in facilities where resuscitation measures are available.”
Professor Stephen Powis, national medical director for the NHS in England, said it was common to have minor incidents during the rollout of a new vaccine.
He said the warning was “precautionary”.
More than 10,000 Brits received the vaccine on the first day, including plucky 91-year-old grandfather Martin Kenyon who rang up his local hospital and demanded a dose.
His interview on American television station CNN has gone viral.
Mr Kenyon has become a global media star following his interview, appearing on Good Morning Britain on Wednesday local time to discuss the jab with Piers Morgan.
“Well it seemed sensible to get on with it because I have two delicious grandchildren and I’ve not been able to hug them for several weeks, months,” Mr Kenyon said.

“I’m planning to spend Christmas with them all. And I thought if I have the vaccine, then I can hug them.”
Mr Kenyon’s interview on CNN had been described as “very British” after he complained about a “nasty lunch” while he was waiting for his vaccine.
He also complained about struggling to get a park when he arrived at the hospital.
The vaccine was currently only being distributed in hospitals, but GPs were taking appointments for bookings from next week.
Britain was likely to have four million vaccine doses before Christmas and authorities were also considering approvals for an Oxford University jab, which was likely in the coming weeks.
Australia will make more than 30 million doses under licence at CSL’s factory in Melbourne if it passes approvals.
Australia has ordered 10 million doses of the Pfizer and BioNTech vaccine, a joint US and German operation, which will be rolled out in March if approved.
Meanwhile, Russian officials warned citizens to avoid alcohol for two months after receiving the country’s COVID-19 vaccine because it weakens their immune system.

VACCINE COULD LAND IN AUSTRALIA EARLY
Prime Minister Scott Morrison hinted that a COVID-19 vaccine could be in Australia before March.
“We are hoping a little bit earlier but that is the current timetable,” he said on 2GB.
“We’ve put our efforts into a number of different vaccines. I mean, in fact, there is no expectation that all of those will come off I mean. That’s why we’ve kept ourselves across a number of them.
“The health side of this is paramount and the TGA must give it the tick off.”
His comments come as a group of campaigning bodies has warned people in poorer countries could miss out on a safe and effective coronavirus vaccine for years to come, accusing richer nations of “hoarding” more doses of COVID-19 shots than they need.
The People’s Vaccine Alliance, an organisation including Amnesty International, Global Justice Now and Oxfam, said that wealthier nations had bought up enough doses to vaccinate their entire populations nearly three times over by the end of 2021.
Canada heads the list with enough doses to vaccinate each citizen five times over, the group said. By contrast, nearly 70 lower-income countries will only be able to vaccinate one in 10 people against the coronavirus next year.

A Reuters report last month said that Canada was in talks with other governments about a plan to donate some COVID-19 doses to lower income-income countries.
The People’s Vaccine Alliance cited data collected by science information and analytics company Airfinity to analyse the deals done between countries and the eight leading coronavirus vaccine candidates. The group said it assumed the coronavirus vaccines currently in clinical trials were all approved for use.
“No one should be blocked from getting a lifesaving vaccine because of the country they live in or the amount of money in their pocket,” Anna Marriott, health policy manager at Oxfam, said in a statement.
“But unless something changes dramatically, billions of people around the world will not receive a safe and effective vaccine for COVID-19 for years to come.”

VACCINE ‘BLACK MARKET’ COULD SPRING UP
As the global population waits in line for the vaccine, a new report suggests that a black market will emerge, benefiting the rich and famous.
In March, when the pandemic first struck the US, entertainers, athletes and the rich were given access to COVID tests while the rest of the country had to wait.
According to a new report in STAT, access to a COVID vaccine will be no different, saying that the rich could fudge the definition of “essential workers” or “high-risk” conditions, lobbying by influential industries, doctors giving in to pressure to keep their patients happy, and even through outright bribery or theft.
“There absolutely will be a black market,” bioethicist Arthur Caplan of New York University told STAT.
“Anything that’s seen as lifesaving, life-preserving, and that’s in short supply creates black markets.”
But US doctor Bill Lang, who was a White House physician during both the Bush and Clinton administrations, disagrees.
“The optics of trying to jump the line would be so bad, they don’t want to do that.” But within the broader system, he said that some people will “inevitably” cut in line.
CANADA APPROVES PFIZER VACCINE
Canada on Wednesday (local time) approved the Pfizer-BioNTech COVID-19 vaccine, days after Britain became the first country to green light and roll it out.
“Today, Canada reached a critical milestone in its fight against COVID-19 with the authorisation of the first COVID-19 vaccine,” Health Canada said in a statement.
The vaccine, it said, had undergone a fast-tracked review while it was still in clinical trials, which concluded that it met “stringent safety, efficacy and quality requirements for use in Canada.”

Prime Minister Justin Trudeau said on Monday that as many as 249,000 doses would be received in December, with the first shipments to 14 sites across Canada arriving as early as next week and people receiving shots a day or two later.
Health care workers and vulnerable populations including the elderly are to be the first to receive it.
By September 2021, Mr Trudeau said, most Canadians should be inoculated. Thousands of Britons became the first in the Western world to receive the vaccine on Tuesday at the start of the biggest global vaccination drive in more than half a century.
The vaccine — which proved to be 95 per cent effective in late-stage clinical trials — is administered in two doses, 21 days apart.
British health officials, however, warned on Wednesday that anyone with a history of significant allergic reactions should not get it right away, after two people suffered allergic reactions and needed treatment.
US giant Pfizer and its German partner BioNTech called Canada’s interim order for its emergency use “a historic moment in our collective fight against the COVID-19 pandemic” and “a major step towards returning to normalcy in Canada.”

They will continue providing data on its use for ongoing evaluation, according to Canada’s health department.
The federal government, meanwhile, has concluded pre-orders with several pharmaceutical companies — including AstraZeneca, Pfizer and BioNTech, Sanofi and GSK, Novavax, Johnson & Johnson, Medicago and Moderna — for 400 million doses, to ensure it eventually gets what it needs for its population of 38 million.
‘V DAY’: EMOTIONAL SCENES AS UK BEGINS COVID JAB
On Tuesday, a 90-year-old woman became the first person in the world to get the Pfizer COVID-19 vaccine outside trial conditions as the UK began its rollout.
Margaret Keenan, who is originally from Enniskillen in Northern Ireland, received the jab at University Hospital in the English city of Coventry.
Mrs Keenan said she felt “so privileged” to be the first person to receive the vaccination, saying it was “the best early birthday present I could wish for” as it would mean she could spend time with her family and friends in the New Year “after being on my own for most of the year”.

“I can’t thank the NHS staff enough who have looked after me tremendously, and my advice to anyone offered the vaccine is to take it – if I can have it at 90 then you can have it too,” she said.
And in a very British twist, the second patient to receive the jab was identified as an 81-year-old man named William Shakespeare, according to the BBC.
Mr Shakespeare of Warwickshire was pictured receiving the vaccine at Coventry’s University Hospital on Tuesday morning (local time).
BREAKING: The 2nd person in the world to have the Covid vaccine is... this is not a joke... WILLIAM SHAKESPEARE from Warwickshire!!! pic.twitter.com/yNbPLXLhfP
— Piers Morgan (@piersmorgan) December 8, 2020
The dose is the first in the UK’s vaccination program that has the promise of saving millions of lives and ending social distancing rules.
Australia has ordered 10 million doses of the Pfizer jab, which if approved by health regulators would be available from March.
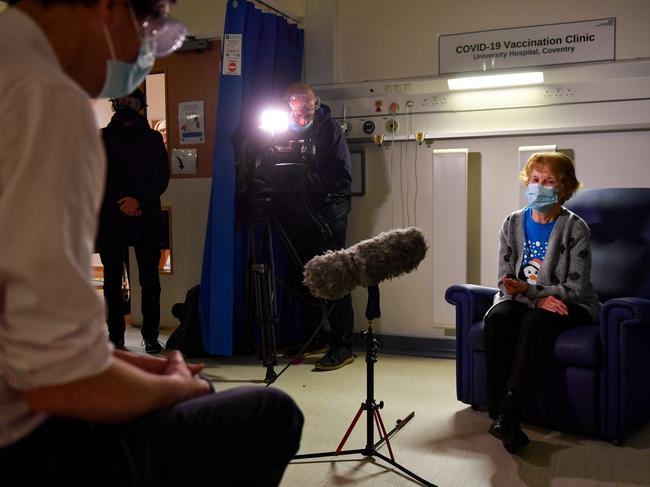
The fanfare surrounding the first dose comes as Britain trials how it will deliver the Pfizer and BioNTech vaccine to millions of people.
There were enough doses available for 400,000 people in the first batch, but the vaccine must be stored at temperatures of up to -70 degrees Celsius.
That was making it hard for people in nursing homes, the most vulnerable, to get access to the jab.
Britain’s National Health Service has scheduled an injection for anyone in the high risk groups coming in for outpatient services this week.
The vaccine must be delivered in two doses 21 days apart, adding an extra layer of complexity to the program.
Britain’s Health Secretary, Matt Hancock, said the vaccination may allow social distancing rules to be lifted within months.
“It has been such a tough year for so many people and finally we have our way through it – our light at the end of the tunnel as so many people are saying,” Mr Hancock told Sky News.
“It seems so simple having a jab in your arm, but that will protect Margaret and it will protect the people around her.

“And if we manage to do that in what is going to be one of the biggest programs in NHS history, if we manage to do that for everybody who is vulnerable to this disease, then we can move on.”
Mr Hancock broke down in tears on Good Morning Britain after announcing there is “light at the end of the tunnel” after Brits received the first jabs on Tuesday morning (local time).
An emotional Mr Hancock, cried as he said it had been “such a tough year”, adding that the vaccine breakthrough made him “proud to be British.”
The UK was the first western country to approve the Pfizer vaccine, ahead of the United States and Europe.
Health regulators were also reviewing results of an Oxford University candidate, a cheaper, easier to store version, which was likely to be approved within weeks.
Russia had approved a jab before clinical trials were completed.

POSSIBLE DATE FOR OVERSEAS TRAVEL REVEALED
International travel bans will remain in place until at least March 17 as the federal government sets out to extend its biosecurity emergency period by another three months.
The move followed advice from the Australian Health Protection Principal Committee (AHPPC) that COVID-19 was an ongoing threat and still posed significant public health risk despite the emergence of the Pfizer vaccine.
Health Minister Greg Hunt said the coronavirus situation was still escalating in many other countries.
“The disease is spreading as quickly as ever,” he said.
“The international world remains a challenging and dangerous environment and Australia won‘t be fully safe until the international community is safe.”

The biosecurity emergency period was set to end on December 17, but the three month extension meant limits would remain on outbound international travel and on international cruise ship arrivals.
Australians can only leave the country with specific exemptions, with Mr Hunt revealing 95,325 exemptions had been granted since the emergency period was first put in place on March 18.
The advice to extend the declaration was accepted by the National Security Committee and will be put to the Governor-General for final approval on Thursday.
Acting Chief Medical Officer Professor Paul Kelly said it was a difficult, but necessary decision.
“We weighed up all of the issues, as the Minister has pointed out, but particularly the ongoing situation internationally and the sort of risks that could come to Australia if we relaxed at this point,” he said.

It comes as the first international flights in nearly six months have touched down in Melbourne this week as part of the state government’s plan to get Aussies stranded overseas home before Christmas.
About 253 passengers among eight international flights arrived on Monday, with health authorities estimating an average of about 160 a day to arrive during the rest of the week.
All of the passengers are taken to the state’s revamped quarantine hotels where they will be tested for COVID-19 and remain for about two weeks.
– with Anthony Piovesan, Zoe Smith, Maria Bervanakis, AFP
More Coverage
Originally published as Coronavirus: US approves Pfizer’s COVID-19 vaccine




