Pfizer and BioNTech apply for emergency authorisation of their vaccine as Oxford jab shows ‘encouraging’ signs
Pfizer and BioNTech have applied for emergency authorisation of their vaccine against coronavirus with limited use to begin next month.
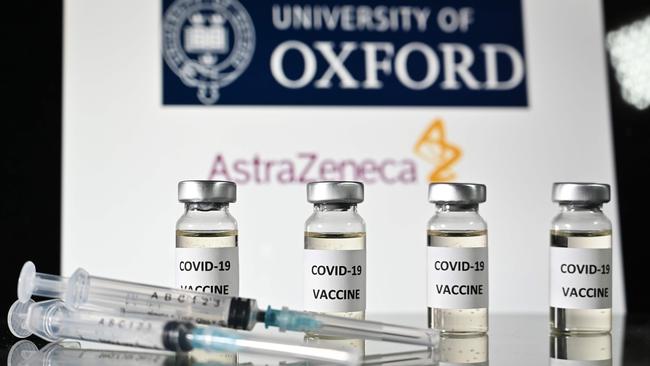
Coronavirus
Don't miss out on the headlines from Coronavirus. Followed categories will be added to My News.
Pfizer and BioNTech said they will ask for emergency approval of their experimental coronavirus vaccine on Friday, local time, a key step that could make the shot available next month.
The Manhattan-based drugmaker and the German biotech firm will be the first to seek a so-called emergency use authorisation in the US for their COVID-19 inoculation, which was 95 per cent effective and posed no serious safety concerns in a major clinical trial.
The vaccine could be distributed to “high-risk populations” in the US by mid- to late December if the Food and Drug Administration approves the request, the companies said in a news release.

“Filing in the US represents a critical milestone in our journey to deliver a COVID-19 vaccine to the world and we now have a more complete picture of both the efficacy and safety profile of our vaccine, giving us confidence in its potential,” Pfizer CEO Albert Bourla said in a statement.
The application boosts US officials’ hopes that they’ll be able to inoculate millions of first responders and other vulnerable people by the end of the year amid a surge in COVID-19 infections and hospitalisation across the country.
Pfizer to seek emergency vaccine OK Friday, doses could ship in 24 hours: Azar
Biotech firm Moderna has said it also expects to seek emergency clearance in the coming weeks for its coronavirus shot, which was nearly 95 per cent effective in a late-stage clinical study.
Federal agencies plan to ship “millions of doses of vaccine within 24 hours of FDA approval,” Health and Human Services Secretary Alex Azar said at a Thursday briefing where he revealed Pfizer and BioNTech’s plans to seek emergency approval.

The companies say they’re working with governments around the world to plan for vaccine distribution while they await approval from regulatory authorities in various countries. The firms have already started the approval process in the UK and plan to submit applications in more regulators worldwide “in the coming days,” they said.
Pfizer expects to have produced up to 50 million doses of its shot globally by the end of the year, while Moderna expects to have about 20 million doses ready to ship in the US.
The vaccines are administered in two doses, suggesting both shots combined could potentially inoculate 35 million people this year.
The US government has ordered 100 million doses of Pfizer’s vaccine for about US$19.50 ($A27) apiece, though Americans will receive the shots for free.
The European Union is planning to order 200 million doses at a cheaper price of 15.50 euros ($A25) per dose, Reuters reported Friday.
OXFORD VACCINE ‘ENCOURAGING’ RESULTS
New tests have shown that Oxford University’s vaccine candidate works in older people and was safe, according to a report in The Lancet.
The findings from the stage two trial were promising, but Oxford researchers were still waiting for the results of the more comprehensive stage three trials of 30,000 people.
Those results were expected within weeks, and if successful, the vaccine could be rolled out in December once government health bodies double check its safety.
Australia has ordered 3.8 million of the jabs to be delivered early in 2021 and will make a further 30 million at CSL’s factories in Melbourne next year.

It comes as the boss of one of the companies leading the charge for a coronavirus vaccine says there is “a light at the end of the tunnel”.
The Oxford researchers did a smaller study of 560 people, which included 240 people over 70.
The stage two study found that older people tolerated the vaccine better than younger people.
Dr Maheshi Ramasamy, investigator at the Oxford Vaccine Group and consultant physician, said the results were a crucial step forward.
“Older adults are a priority group for COVID-19 vaccination, because they are at increased risk of severe disease, but we know that they tend to have poorer vaccine responses,” she said.
“We were pleased to see that our vaccine was not only well tolerated in older adults, but also stimulated similar immune responses to those seen in younger volunteers.
“The next step will be to see if this translates into protection from the disease itself.”
The Oxford vaccine has been one of the most ordered, partly because it can be kept in normal fridges and uses existing vaccine technology and was one of the early frontrunners.
However, the stage three trial was halted because one volunteer had spinal inflammation.
That was later ruled to not have been caused by the vaccine.
Pfizer and BioNTech’s groundbreaking vaccine uses mRNA, which needs to be stored at -70 degrees.
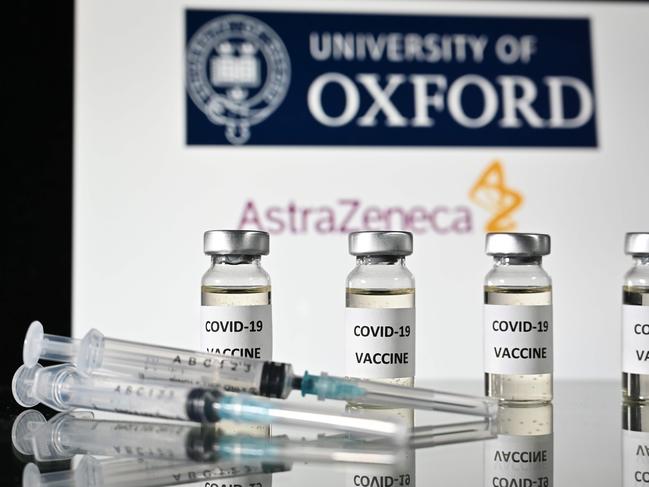
Australia has ordered 10 million doses of the Pfizer vaccine.
A Moderna vaccine, which announced results this week, can be stored in normal freezers and fridges, but Australia has yet to order that jab.
The average age of patients who have died with coronavirus was 82.4 in the UK.
More than 52,000 of the 58,000 who have died of the virus have been over the age of 65, according to the UK’s Office for National Statistics data on Thursday.
The results mean there is a chance that the vaccine would be able to slash death tolls if pensioners were vaccinated first.
Professor Andrew Pollard, from the University of Oxford said he was “absolutely delighted” with the latest trial results.
“The other thing that we found which I think is really important is the vaccine is really well tolerated in those who are over 55,” he told the BBC.
“We do know with these vaccines that adults tend to feel a bit ropy the day after they have been vaccinated … but that was very, very much less, particularly in those who are over 70.
THE STAR WHO HELPED FUND THE FIGHT AGAINST THE VIRUS
Meanwhile, superstar Dolly Parton has been revealed as a major donor to Pfizer rival Moderna’s effort to find a vaccine.
Parton is being hailed as a COVID “saviour” after donating $A1.4 million to help fund the Moderna vaccine.
The iconic country singer, 74, donated the cash back in April to Vanderbilt University Medical Centre in Nashville, Tennessee, for coronavirus research.
This week, US company Moderna announced its vaccine may be 94.5 per cent effective against the killer respiratory disease and Parton is namechecked in the preliminary report, The Sun reports.

Published in the New England Journal of Medicine, the report states that the work was supported by the “Dolly Parton COVID-19 Research Fund” among other groups.
The glamorous music star became friends with Dr Naji Abumrad of the Vanderbilt Institute when he treated her in 2014 following a car crash.
Earlier this year, the scientist told Parton that his team was making “some exciting advancements” in the search for a cure of the virus.
She then donated $1m to help fund the research and encouraged other wealthy people on Twitter to do the same.
The singer told the BBC: “I’m sure many, many millions of dollars from many people went into that (research fund) but I felt so proud to have been part of that little seed money that hopefully will grow into something great and help to heal this world.
“Lord knows we need it!”
CONTRACT DELAY COULD STALL VACCINE DELIVERY
The CEO of US biotech company Moderna warned European countries on Tuesday (local time) that dragging out negotiations to buy its promising COVID-19 vaccine will slow down deliveries, as other nations that have signed deals will get priority.
“It is clear that with a delay this is not going to limit the total amount but it is going to slow down delivery,” Moderna CEO Stephane Bancel said.
Moderna announced this week that its experimental vaccine is almost 95 per cent effective in protecting people from the coronavirus, further boosting hopes of an end to the pandemic after Pfizer released similar findings last week about its vaccine.
In coming weeks both are expected to ask American, European and other authorities for approval to roll out their vaccines for widespread use, as the world reels from another surge in COVID-19 cases.
Around the globe the pandemic has claimed more than 1.3 million lives, and reported infections top 55 million.
The United States is the worst hit in both categories in absolute terms, as fatalities approach 250,000 and the total caseload exceeds 11 million.
Mr Bancel said the US has already reserved 100 million doses since early August and several million doses are now in storage awaiting US regulatory approval, probably in December.
Over the summer Moderna engaged in discussions with the European Commission, the executive arm of the EU, about it buying 80 million doses of the vaccine but no contract has been signed, Mr Bancel said from the company headquarters in Cambridge, Massachusetts.
AMERICANS ‘MOST EAGER’
In the meantime Moderna, which developed its product in conjunction the National Institutes of Health in the US, has signed agreements to provide the vaccine to Canada, Japan, Israel, Qatar and Britain, he said.
Mr Bancel said there is much red tape involved in getting a vaccine approved, complicated by the EU having 27 member nations.

By contrast, it took just two weeks to cement a deal on providing the vaccine to Canada, from the time the two sides began talks to the signing of a contract.
If EU medical regulatory authorities approve the Moderna vaccine by the end of the year but no contract has been signed, the EU will not be among the first to receive it.
“So we will get started in Switzerland, it will get started a little in Japan, Israel, Canada — in the countries that have placed orders. But I will not be able to send product to countries that have not placed orders.” said Mr Bancel.
“The longer they wait, the longer it will take,” he told said, adding that the price is not an issue in talks with the Europeans, and declining to comment further on the negotiations.
The European Commission, which has signed deals with Pfizer and other manufacturers, said the negotiations were highly complex.
“Are we simply concluding contracts because all of a sudden, there are some nice press reports about the status of this vaccine? That is clearly not the case,” commission spokesman Stefan de Keersmaecker said Tuesday at a briefing.
The Americans on the other hand have been most eager, Mr Bancel noted. As early as March 2, said Mr Bancel, he and other Big Pharma executives were sitting in the White House in a meeting with US President Donald Trump.

HOW COVID WILL FAST-TRACK VACCINES FOR OTHER DISEASES
Scientists are confident they will now be able to fast track vaccines against any new disease after the success of a second COVID-19 vaccine using new age mRNA technology.
Instead of having to go through the lengthy process of deactivating a pathogen to make a vaccine all they need now to make a vaccine is a fragment of the virus’ genetic code.
US company Moderna cemented the success of this new method when it reported clinical trials showing its COVID-19 vaccine is 94 per cent effective at preventing COVID-19.
Like the first successful COVID-19 vaccine made by Pfizer, Moderna’s uses a radical new technology called mRNA.
To make the vaccine researchers use a piece of the coronavirus’ genetic code called messenger RNA (mRNA) to train the body to make the virus’ spike protein.
This prompts the immune system to recognise and respond when it comes across the virus.
Conventional vaccines usually contain inactivated disease-causing particles of the virus to stimulate an immune response and can take some time to produce.
The mRNA technology is a new way of making vaccines that is much faster and cheaper and it holds tremendous potential.
No vaccines using this method have ever been used in humans before but now we have two clinical trials suggesting the method actually works very well.
The speed with which these vaccines were produced shows they have the edge over more conventional methods of vaccine production.
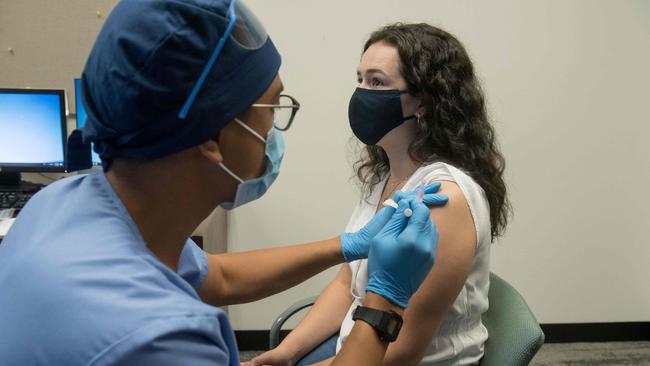
Dr Zoltán Kis from the Future Vaccine Manufacturing Hub at Imperial College London, said the beauty of this technology was that “vaccine candidates can be produced against virtually any disease”.
“This is a platform technology, meaning that the production process, the purification processes and the formulation processes can remain the same no matter what vaccine or vaccine candidate is produced,” he said.
This is a huge advantage in terms of the speed at which vaccine candidates can be developed and produced in the future, he said.
“With conventional vaccine production technologies, each vaccine required the development of a new production process, whereas in the case of the RNA vaccine production platform technology one process can produce vaccines and vaccine candidates against virtually any disease,” he said.
Moderna’s vaccine can be stored in a normal refrigerator which gives it a huge advantage over the successful Pfizer vaccine which needs to be stored at temperatures below minus 70 -80 degrees.
It has been tested in the elderly and well as people from ethnically diverse backgrounds.
While it prevents people from getting COVID-19 – the illness caused by the virus – it is unclear whether it actually prevents the spread of the virus.
Australia does not have a direct deal to purchase the Moderna vaccine. However, we have signed up to the Covax initiate, an international agreement for vaccine supply which will eventually give us access to this vaccine.
Moderna says it can make 20 million doses of the vaccine by the end of the year. It aims to produce 500 million doses next year, with the possibility of 1 billion doses if it can obtain enough raw materials.
MORE NEWS
How the Pfizer and Moderna vaccines compare
COVID-19 vaccines selling for $24,000 on black market
Every parent and kid to get a mental health check
Originally published as Pfizer and BioNTech apply for emergency authorisation of their vaccine as Oxford jab shows ‘encouraging’ signs


