Medicare coverage advances for Lumos’ FebriDx point-of-care respiratory test
Lumos secures reimbursement coverage for FebriDx from two additional Medicare Administration Contractors in the US.

Stockhead
Don't miss out on the headlines from Stockhead. Followed categories will be added to My News.
Lumos secures reimbursement coverage for FebriDx from two additional Medicare Administration Contractors
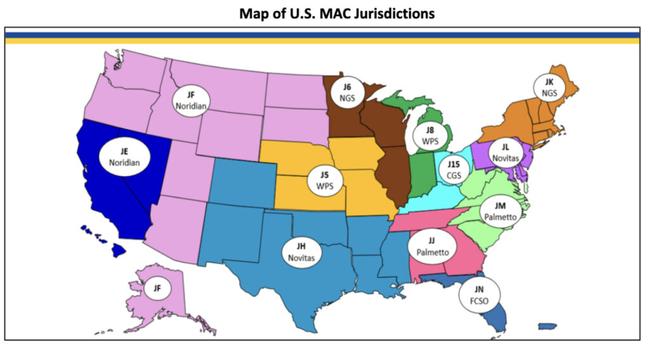
US reimbursement coverage now achieved with four of seven Medicare Administration Contractors
Momentum continues with negotiations in process with remaining three
Special Report: Lumos Diagnostics is continuing to advance the US reimbursement pathway for its FebriDx test, a rapid point-of-care diagnostic designed to differentiate between bacterial and non-bacterial acute respiratory infections.
Lumos Diagnostics (ASX:LDX) has announced it has received confirmation of Medicare reimbursement coverage from two additional Medicare Administrative Contractors (MACs), First Coast Service Options (FCSO) and Noridian Healthcare Solutions (Noridian).
The company said FebriDx would be added to the Medicare fee schedules at of FCSO and Noridian, with coverage backdated to April 1, 2025.
FCSO is responsible for managing Medicare payments in the state of Florida. Noridian provides coverage across a wide geographic region that includes California, Nevada, Oregon, Washington, Idaho, Montana, Utah, Arizona, Wyoming, North Dakota, South Dakota, Alaska and Hawaii.
The inclusion of the two MACs adds to coverage from Novitas and Palmetto announced last week.
Lumos said it now had Medicare reimbursement from four of the seven MACs, representing over 55% of the total US Medicare payment coverage.
Pic: Lumos Diagnostics
Set reimbursement rate for FebriDX
Coverage by the MACs follows the Centers for Medicare & Medicaid Services (CMS) including FebriDX in the 2025 Clinical Lab Fee Schedule (CLFS).
Under the CLFS, a proprietary laboratory analyses (PLA) code (0442U) was set for FebriDX to be reimbursed at a rate of US$41.38 per test from January 1.
Lumos said the CMS set PLA code was a pivotal milestone in securing reimbursement for FebriDx from both government and private insurers.
Momentum in its commercialisation efforts significantly expands Lumos’ access to the Medicare patient population.
Medicare adoption often sets a precedent for private insurers, paving the way for broader reimbursement acceptance.
The company said reimbursement coverage, particularly from private payors, traditionally followed real-world use.
Addressing antibiotic resistance
FebriDx enables point-of-care differentiation between bacterial and non-bacterial infections – a critical capability given that most acute respiratory infections are viral in nature and do not require antibiotics.
Despite this, antibiotics are still prescribed in up to 50% of these cases, fuelling the growing challenge of antibiotic resistance.
The World Health Organization has warned that the global rise in antibiotic resistance poses a major threat to public health, reducing the effectiveness of commonly used antibiotics against widespread bacterial infections.
Managing director Doug Ward said securing additional Medicare coverage from FCSO and Noridian for FebriDx reinforces its commitment to developing a POC diagnostic solution that benefits patients, but also integrates seamlessly into care pathways and delivers value to the broader health system.
“Lumos remains committed to securing adoption from the remaining three MACs and pursuing reimbursement coverage from private payors, with the goal of achieving nationwide access for patients and supporting broad clinical adoption of FebriDx,” he said.
This article was developed in collaboration with Lumos Diagnostics, a Stockhead advertiser at the time of publishing.
This article does not constitute financial product advice. You should consider obtaining independent advice before making any financial decisions.
Originally published as Medicare coverage advances for Lumos’ FebriDx point-of-care respiratory test


