Shane Cook fights for justice after Logan Hospital mesh implant ended in three years of hell
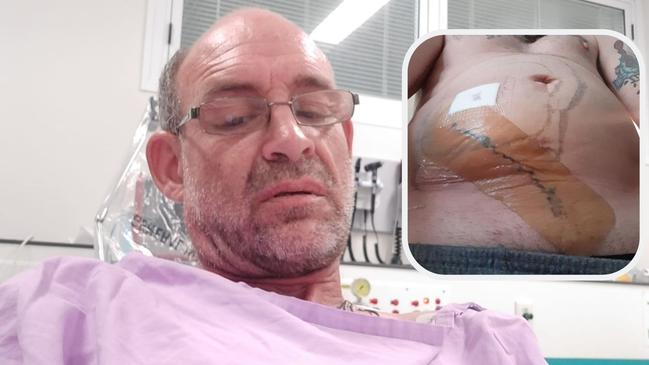
A man who begged for three years to have a surgical implant removed after having a sensation of ants crawling around in his abdomen, is calling for a full audit of the product in Australia.
Logan
Don't miss out on the headlines from Logan. Followed categories will be added to My News.
A man who begged for three years to have a surgical implant removed after having a sensation of ants crawling around in his abdomen, is calling for the product to be banned in Australia.
Shane Cook, 48, pleaded with doctors at Logan Hospital to remove a surgical mesh he had inserted in 2016 to fix a hernia in his groin.
His requests were overruled despite the Therapeutic Goods Administration registering six “adverse event” notifications about Parietex Composite Mesh before Mr Cook had his 2016 operation.
A further three reports were made after his surgery with the TGA recording 21 incident reports regarding nine different types of Parietex mesh since 2012.
The TGA issued a hazard alert in 2018 and then recalled and banned a similar Parietex Composite Parastomal mesh device from use in Australia.
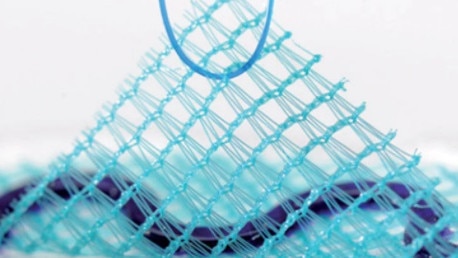
In 2018, a Senate committee also looked into pelvic mesh implants and found the quality of life of women who had received the implants was significantly diminished.
But despite the adverse TGA reports and going to Logan Hospital’s emergency department in agony 30 times and two exploratory operations, doctors refused to remove the mesh, claiming it was doing its job.
A Logan Hospital doctor told Mr Cook the mesh was not the cause of his pain and no further surgery was required but painkillers were prescribed.
It was a recommendation from a Sydney herniaologist that backed up Mr Cook’s right to have the mesh removed.
Mesh expert and diagnostic surgeon Dr John Garvey said Mr Cook’s pain was “a typical example of mesh inguinodynia” and told his Logan GP the mesh had to be removed either laparoscopically or via laparotomy”.
The surgery was conducted at Queen Elizabeth II Hospital in 2019 and months after the operation, Mr Cook reported feeling better.
Now the father of two, who cannot work and is on a disability pension, said he was ready to take his fight for justice further.
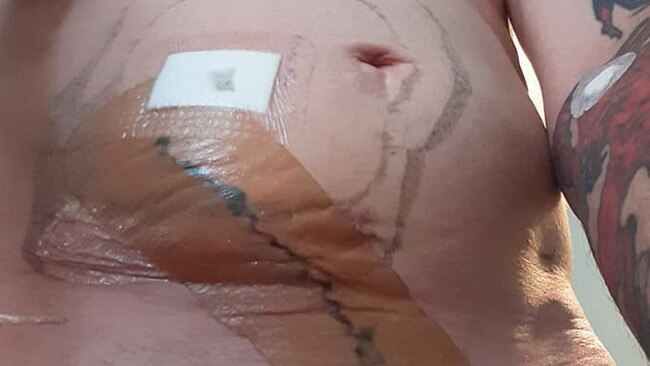
“The Logan Hospital sold the mesh to me like it was a new car, telling me that it was the latest and greatest treatment for hernia repairs and had excellent results in preventing reherniation,” Mr Cook said.
“I was never told the full story about the mesh or what trials had been done or that if there were complications doctors couldn’t remove it.
“The TGA approved these implants without conducting any post market surveillance.
“These are permanent implants and there is no registry created to report and monitor adverse effects of these implants and there are only four surgeons in Australia skilled enough to remove them.”
Queensland Health, which oversees Logan Hospital, said it could not comment on individual cases but, like other states and territories, followed the advice of the TGA.
Mr Cook took his complaint to the TGA, which said it conducted a review of his case and found no action would be taken.

The letter, which was signed electronically by an unnamed administration officer, gave no reason for not pursuing the matter.
“In this instance, no further investigation of the reported event will occur,” the unnamed TGA officer said.
“The TGA will continue to monitor the rate and pattern of occurrence of the reported adverse event and may re-open the file as appropriate.”
A Queensland Health Ombudsman report included the professional opinion of Brisbane surgeon Professor Kellee Slater, who said Logan Hospital did not take appropriate steps to mitigate Mr Cook’s pain.
“It is my opinion that this patient should not have had a hernia repair as a treatment for the pain,” she wrote in the submission.
“Mr Cook was also not treated with an acceptable level of care following his surgery.
“He had multiple presentations to the emergency department for pain and treatment of this pain with narcotics resulted in narcotic dependence.
“Mr Cook should have been seen in surgical outpatients urgently by the consultant surgeon who performed the surgery after his first representation to the emergency department.
“While Logan is a teaching hospital, any patient who deviates from a normal post-operative course should be escalated to the consultant to manage to avoid the protracted pain and suffering that Mr Cook experienced.”

Attorney-General Shannon Fentiman also took up Mr Cook’s plight and called on the Therapeutic Goods Administration to conduct a full review of surgical mesh.
Ms Fentiman also asked for an audit of all patients who have received the products and for a high-risk implantable device registry.
“Shane was plagued with chronic pain that was only alleviated after by the removal of the implant,” Ms Fentiman wrote in a letter to the federal Medical Devices Surveillance branch last year.
The Therapeutic Goods Administration said it would not cancel the product’s registration and said there had been no deaths from the product in Australia.
“Based on our assessment of the incident and the information submitted by the sponsor, the TGA did not take any regulatory action,” the TGA said.
“We continue to actively monitor signals of any devices that have an altered benefit-risk profile and will act accordingly upon review of the clinical and scientific evidence to safeguard patients from harm.”
Medical technology company Medtronic said there had been extensive clinical data to support the use of Parietex meshes and no product would be sold in Australia without TGA approval.
“We recently sold our one millionth piece of Parietex composite mesh, which has more than 15 years of documented clinical and preclinical effectiveness,” Medtronic said.
“Prior to the approval of a device being sold in Australia, the TGA reviews all relevant evidence to determine whether the benefits of the device outweigh any possible risks.
“All our implantable medical devices are supplied with instructions for use … we have a commitment to ensure product labelling is updated with the latest clinical and safety information.”
More Coverage
Originally published as Shane Cook fights for justice after Logan Hospital mesh implant ended in three years of hell





