US Medicare coverage grows for Lumos’ FebriDx point-of-care respiratory test
Lumos secures reimbursement coverage for its FebriDx point-of-care respiratory test from a fifth US Medicare Administration Contractor.

Stockhead
Don't miss out on the headlines from Stockhead. Followed categories will be added to My News.
Lumos secures reimbursement coverage for FebriDx from another US Medicare Administration Contractor
US reimbursement coverage achieved with five of seven Medicare Administration Contractors
Medicare adoption generally serves as precedent for reimbursement decisions by private insurance payors
Special Report: Lumos Diagnostics has received confirmation of Medicare reimbursement coverage from another US Medicare Administrative Contractor (MAC) for its FebriDx test, a rapid point-of-care (POC) diagnostic designed to differentiate between bacterial and non-bacterial acute respiratory infections.
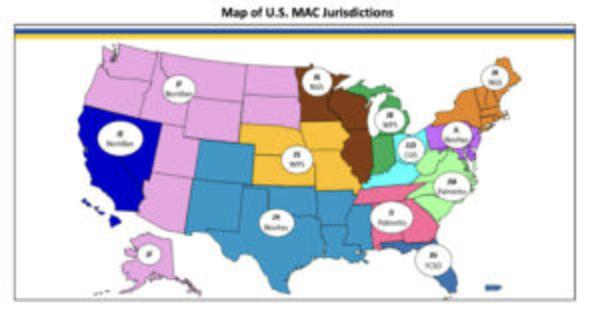
Lumos Diagnostics (ASX:LDX) said FebriDX would be included in the Medicare fee schedule of MAC WPS Health Solutions (WPS), which is responsible for managing Medicare payments in the states of Iowa, Kansas, Missouri, Nebraska, Indiana and Michigan.
With the inclusion of WPS, Lumos now has Medicare reimbursement from five of the seven MACs for FebriDx, representing more than 70% of the total US Medicare payment coverage.
The company secured coverage in April from Novitas and Palmetto, First Coast Service Options and Noridian Healthcare Solutions, significantly expanding access to the US Medicare patient population for FebriDx.
Coverage by the MACs follows the Centers for Medicare & Medicaid Services (CMS) including FebriDX in the 2025 Clinical Lab Fee Schedule (CLFS).
Under the CLFS, a proprietary laboratory analyses (PLA) code (0442U) was set for FebriDX to be reimbursed at a rate of US$41.38 per test from January 1.
Lumos said coverage by WPS at US$41.38 per test would be backdated to May 1, 2025.
Source: Lumos Diagnostics
Reimbursement critical validation milestone
FebriDx enables point-of-care differentiation between bacterial and non-bacterial infections – a critical capability given that most acute respiratory infections are viral in nature and do not require antibiotics.
Despite this, antibiotics are still prescribed in up to 50% of these cases, fuelling a growing challenge of antibiotic resistance
Lumos said achieving reimbursement coverage by the MACs was a critical validation milestone on the pathway to meaningful adoption of FebriDx in the US.
Medicare adoption often sets a precedent for private insurers, paving the way for broader reimbursement acceptance.
Lumos said over time, commercialisation success for FebriDx would be driven by demonstrating three essential outcomes including:
- Clear clinical benefit – evidence that the test improves patient care
- Impact on the clinical care pathway – showing that the test makes the diagnostic and treatment process more efficient or effective
- Favourable economics – proving that the test is financially beneficial for the healthcare system.
“Securing additional Medicare coverage from WPS for FebriDx reinforces our commitment to developing a point-of-care diagnostic solution that not only benefits patients, but also integrates seamlessly into care pathways and delivers value to the broader health system,” managing director Doug Ward said.
“Lumos remains committed to securing adoption from the remaining two MACs and pursuing reimbursement coverage from private payors, with the goal of achieving nationwide access for patients and supporting broad clinical adoption of FebriDx.”
This article does not constitute financial product advice. You should consider obtaining independent advice before making any financial decisions.
Originally published as US Medicare coverage grows for Lumos’ FebriDx point-of-care respiratory test


