Coronavirus: Pfizer to test COVID vaccine in young children; EU faces worsening vaccine war
Pfizer has commenced clinical trials for its COVID vaccine in children as young as six months old, as the EU faces a worsening vaccine war.
World
Don't miss out on the headlines from World. Followed categories will be added to My News.
Pfizer has begun clinical trials for its COVID vaccine in children under the age of eleven, an early sign of the next stage of the global immunization campaign.
“Together with our partner BioNTech, we have dosed the first healthy children in a global Phase 1/2/3 continuous study to further evaluate the safety, tolerability, and immunogenicity of the Pfizer-BioNTech COVID-19 vaccine,” the company said in a statement.
“We are proud to start this much needed study for children and families eagerly awaiting a possible vaccine option.”
According to details posted on the site clinicaltrials.gov, the company is testing three different dosing levels for use in this age group.
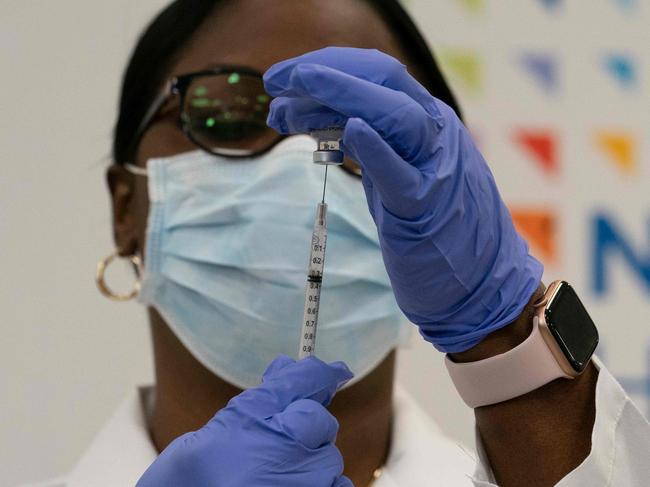
Children aged six months to two years; two years to five years, and five years to 11 years are taking part in the global study that has 144 participants.
The company is already testing the shots in children aged 12 to 15, and its US emergency authorization covers people aged 16 and up.
Pfizer joins Moderna and AstraZeneca in testing their vaccines in younger children, while Johnson & Johnson has plans to follow.
While children are generally spared the worst of the disease and are less likely than adults to transmit the virus, rare cases of serious Covid and deaths do occur, as well as a post-infectious inflammatory condition called MIS-C.
Under-18s account for roughly a fifth of the US population of 330 million, and most experts believe it will be necessary to make inroads towards immunizing children in order to achieve population level immunity.
EU’S VACCINE WAR, ASTRAZENECA FACES NEW SCRUTINY
The European Medical Agency (EMA) has announced it will convene a group of experts on Monday to delve further into incidents of blood clotting in people who have received AstraZeneca’s COVID-19 vaccine.
It comes as EU leaders debated how to rescue their chaotic coronavirus vaccination drive and secure millions more doses as a third wave of infections breaks over the bloc.
The Amsterdam-based regulator last week declared the jab was “safe and effective” and not linked to clotting.
The group of medical experts and two representatives of the public will “provide additional input into the assessment”, the EMA said in a statement on Thursday (local time).
Numerous European countries quickly followed the EMA recommendation and lifted their suspensions of AstraZeneca vaccinations as infections and deaths continue to climb.
The agency declared, as did the World Health Organisation, that the benefits from the jab continue to outweigh the risk of side effects.

But the EMA is pursuing investigations, noting a link could not definitively be ruled out to a rare clotting disorder.
The group convening on Monday will cover “aspects such as any plausible mechanism of action, possible underlying risk factors and any additional data needed to gain a deeper understanding of the observed events and the potential risk”, an EMA statement said.
The outcome of the expert meeting will feed into the safety panel’s updated health recommendation due to be published between April 6-9.
Beyond production and supply issues, AstraZeneca has faced other challenges that have dented confidence.
The pharma giant was forced to review its US trials and then slightly revise down the jab’s efficacy from 79 per cent to 76 per cent, after an American agency raised concerns about outdated information.
Top US infectious disease expert Anthony Fauci had said the discrepancy was a “bump in the road” and that data would show it is “a good vaccine”.
Where the EU vaccine rollout has stuttered, there has been huge progress elsewhere.
Israel said Thursday more than half of its 9.2 million people have received both doses of the Pfizer/BioNTech shot after it sped ahead with its vaccine drive.
And in the United States — the world’s worst-hit nation — around 70 per cent of Americans aged 65 or over have received at least one dose.

EU’S VACCINE CHAOS
EU leaders debated Thursday (local time) how to rescue their chaotic coronavirus vaccination drive and secure millions more doses as a third wave of infections breaks over the bloc.
US president Joe Biden was due to dial in for a cameo appearance later in the EU video summit, but even his star power couldn’t mask the danger.
The 27 members have banded together behind the European Commission in a joint purchasing strategy — but actual vaccination rates have lagged behind those in the US and UK.
Host Charles Michel, president of the European Council, had hoped to hold a face-to-face summit but was forced to accept a stripped-down video conference because of travel curbs.

Some European countries are reintroducing stricter lockdown rules to head off a new tide of cases, but the bloc is divided on how to share out vaccine supplies and respond to what they see as unfair British tactics.
A key summit topic is the European anger over UK-based pharma giant AstraZeneca failing to meet vaccine delivery promises while ensuring smooth supplies to former EU member Britain.
As the leaders talked, European Commission chief Ursula von der Leyen revealed that companies that produce in the EU had exported 77 million does outside the bloc since December.
Of these doses, 21 million went to the UK — two thirds of the total administered there so far.
The figures were intended as a rebuke to Britain, which has criticised the EU for “vaccine nationalism” in tightening controls on exports.
EU countries are not all happy with a beefed-up European Commission move that could block some vaccine shipments to countries like Britain which produce jabs but don’t export them.
But draft conclusions support the EU export authorisation scheme, while urging Europe to step up vaccine production.

“We underline the importance of transparency as well as of the use of export authorisations,” the draft says.
“We reaffirm that companies must ensure predictability of their vaccine production and respect contractual delivery deadlines.” French President Emmanuel Macron admitted on the eve of the summit that Europe had lacked ambition while the United States, in particular, forged ahead with its inoculation drive.
“We weren’t quick enough, strong enough on this,” he told Greece’s ERT television. “We thought that the vaccine would take time to take off.”

PRESSURE ON ASTRAZENECA
A European source described the EU export authorisation mechanism as “a loaded gun under the table”.
It is widely seen as a means to pressure AstraZeneca to boost deliveries.
But Ireland, Belgium and the Netherlands are among countries wary of any move to block exports from vaccine producers such as Pfizer/BioNTech, which supplies both the EU and UK.
Von der Leyen’s immediate predecessor, former commission chief Jean-Claude Juncker, was scathing about the strategy.
The EU “used to be the world free trade champion, so I don’t think that this is the right way to go,” he told the BBC.

“I do think that we have to pull back from a vaccine war.” If global vaccine supply chains are disrupted, many countries could lose out, as both British Prime Minister Boris Johnson and von der Leyen accept.
They are in discussions about how “to create a win-win situation and expand vaccine supply for all our citizens” but have yet to agree on how to share AstraZeneca doses.
The firm is expected to deliver 30 million doses to the EU in the first quarter — a pledge already radically reduced from a contractual 120 million doses.
Much of the focus of Brussels and London is on an AstraZeneca plant in the Netherlands producing doses which both sides claim should be theirs.
A European diplomat told reporters that an acceptable compromise would be the UK and the Commission agreeing to equally shoulder the AstraZeneca shortfall.
VACCINE WAR?
Another sensitive issue is the distribution of vaccines which Europe has already received.
A group of smaller states led by Austria is demanding more vaccines after they missed an earlier opportunity to secure a bigger share of costlier versions by betting on the cheaper — but unreliably supplied — AstraZeneca one.
“In the spirit of European solidarity, a fair distribution of vaccine doses within the EU is needed in order to avoid a Europe of two classes in vaccination,” Austria’s Chancellor Sebastian Kurz tweeted.
But there was little sympathy among other delegates for Kurz’s tactics, which were seen as trying to blame Brussels for Austria’s failure to make good use of the existing sharing mechanism.

Against this backdrop, Mr Biden’s appearance offers a positive note, with European officials delighted with the new US administration’s warmer tone.
Just before the summit started, Mr Biden’s top diplomat Antony Blinken wrapped up a two-day visit to Brussels that included talks with NATO ministers and top EU officials in which close co-ordination was pledged.

BIDEN ANNOUNCES 200m GOAL
Meanwhile, Mr Biden on Thursday (local time) launched a new goal of administering 200 million doses of COVID-19 vaccine in the United States within his first 100 days in office, double his original pledge.
“Today I’m setting a second goal, and that is, we will by my 100th day in office have administered 200 million shots in people’s arms,” Mr Biden told reporters in his first press conference since taking office on January 20.
“I know it’s ambitious, twice our original goal,” he added. “But no other country in the world has even come close — not even close — to what we are doing, and I believe we can do it.”
More Coverage
Read related topics:Donald Trump



