Budget 2021: Alison Day meets with Greg Hunt to help other cancer sufferers
Mum-of-two Alison Day - who has a terminal breast cancer diagnosis - has secured more funding in the budget for those fighting aggressive forms of the disease.
NSW
Don't miss out on the headlines from NSW. Followed categories will be added to My News.
The dying wish of a brave Sydney woman seeking to help others battling aggressive breast cancer has been heard by the federal government, with funding for clinical trials and support for faster access to new drugs in the Budget.
After Alison Day learned she would not survive her triple negative breast cancer diagnosis, she began lobbying for expedited access to a promising drug called Trodelvy, and for other women to receive molecular testing that would connect them with new treatments and trials.
Following the Ali’s Wish campaign, The Daily Telegraph can reveal today’s Federal Budget will include $5 million to support a clinical trial of potential new drugs for the treatment of refractory low survival breast cancer in women who have been identified through molecular testing.

Health Minister Greg Hunt has also personally appealed to Trodelvy manufacturer Gilead, with the company committing to apply for listing on the Pharmaceutical Benefits Scheme to treat breast cancer in July, with a determination expected by November.
While only 15 per cent of breast cancer patients are diagnosed triple negative, the aggressive disease has a five year survival rate of about 77 per cent, which is significantly worse than other types.
Ms Day welcomed the trial funding announcement, even though it would not save her own life.
“I believe had I been diagnosed five years from now, in a world where molecular testing and expedited approval processes to oncology drugs with high clinical need was ‘the norm’ I would have survived,” she said.
“It’s my hope that for those diagnosed in the future, things will be different and this is a great first step.”
As she fought to have as much time with her two daughters, aged 10 and 17, as possible, Ms Day last month travelled to Melbourne where she met with Mr Hunt to make her case.
“I shared my story with Mr Hunt in order to highlight the experience of many women with low survival breast cancer, including triple negative breast cancer, many of whom only have access to standard chemotherapy, radiation and surgical options for their treatment,” she said.
“While this is effective for many, unfortunately 30 per cent of triple negative patients will receive treatment and still have a recurrence of the disease, often leading to metastatic disease and ... death.”
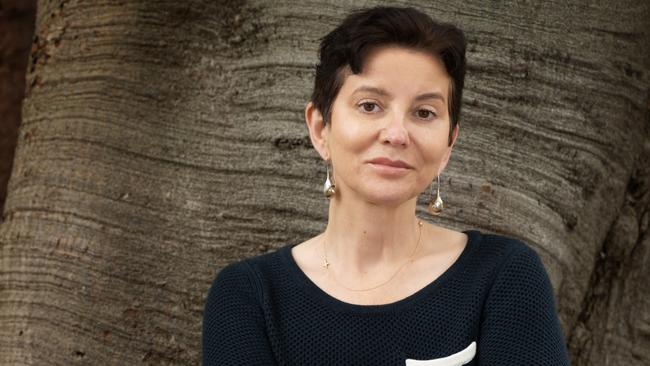
After the meeting, Mr Hunt wrote to Gilead chief executive Jaime McCoy to formally invite the company to immediately apply to have Trodelvy listed on the PBS, revealing he had been “deeply moved” by the advocacy of Ms Day on behalf of Australian cancer patients.
He said while Trodelvy was currently being evaluated by the Therapeutic Goods Administration, many patients would not be able to access the drug until it was subsidised on the PBS.
“TGA registration is a critical step towards access in Australia, (but) the cost of this medicine is prohibitively expensive for Australian patients,” he wrote.
“Therefore, as an outcome of recent positive discussions with your team, I am writing to you to formally invite you to expedite an application to the Pharmaceutical Benefits Advisory Committee (PBAC) so that the government could action a listing for this medicine on the PBS at the earliest opportunity, should it be recommended by PBAC.”

Mr Hunt said the government had an “iron clad” commitment to list all medicines recommended by the PBAC for subsidy.
“Should Trodelvy receive a favourable recommendation from these independent medical experts, the government will list this as a matter of priority,” he said.
The $5m for breast cancer trials will come from the government’s Medical Research Future Fund, and applications will open immediately on Tuesday and close on July 7.
It is hoped that this funding will uncover new drugs, or new uses for existing drugs, to improve patient outcomes.
Mr Hunt said he believed molecular testing would be a standard of care for all cancer patients in Australia within the next decade.

His meeting with Ms Day and personal appeal to Gilead followed considerable advocacy from Australian women battling triple negative breast cancer, and came at a time when the government has been criticised for failing to listen to women.
In the past three months the government was rocked by allegations of sexual assault and poor treatment of women in the federal parliament, with today’s Budget widely expected to address public concerns over the issue.
The Budget will include $100.4m over four years to improve cervical and breast cancer screening and $95.9m for new tests for preimplantation genetic testing of embryos for specific genetic or chromosomal abnormalities before pregnancy.
In other Budget moves, a doubling of funding for domestic violence prevention is expected, while the childcare subsidy will rise to 95 per cent for the second and subsequent child aged under five, in a bid to get more women back into the workforce.
Single parents with dependent children will also be able to apply for a home loan with a deposit of only two per cent, as the government would go guarantor on the remaining amount.
Finance Minister Simon Birmingham insisted the investment in these areas was unrelated to the recent scrutiny over women's’ issues.
Read related topics:Federal Budget 2021




