Australia to subsidise multiple myeloma drug Darzalex under PBS
Blood cancer patients will be among those to benefit with better access to medicine under the Pharmaceutical Benefits Scheme.

NSW
Don't miss out on the headlines from NSW. Followed categories will be added to My News.
New hope is on offer for patients with the deadly blood cancer multiple myeloma with the subsidised listing on the Pharmaceutical Benefits Scheme of the treatment Darzalex, or daratumumab come November 1
Until now, Australians with multiple myeloma have received Darzalex by infusion which typically takes several hours to administer per month in a hospital or infusion centre but the new listing is for the treatment to be injected in 3-5 minutes saving patients an estimated 70 hours a year for treatment.
Professor Miles Prince from the Peter MacCallum Cancer Centre says Darzalex SC came at a time when it was important for patient to limit their time in hospital to reduce potential exposure to Covid-19.
“Less time spent in hospital will also be an advantage for many cancer patients who are doing their best to reduce potential exposure to Covid-19,” Professor Prince said.
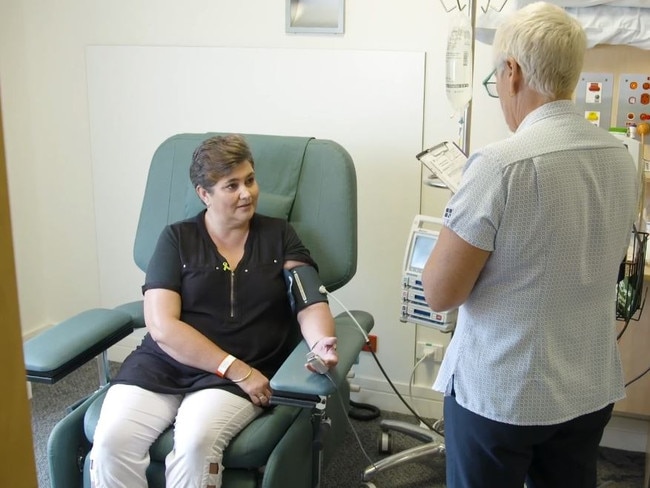
Multiple myeloma patient, Maria Camerotto from Melbourne, was the first patient in Australia to receive Darzalex SC on compassionate grounds in July this year, as her clinicians were unable to access her veins to administer intravenous.
Without this subsidy, patients would pay about $150,000 for the first year of treatment with Darzalex SC.
An estimated 1,000 Australians will be eligible for treatment with Darzalex SC each year. 6 Eligible patients will now pay $41.30 (general patients) or $6.60 (concessional patients) for each phase of treatment.
Got a news tip? Email weekendtele@news.com.au