Prof Nikolai Petrovsky to start human trials of Alzheimer’s vaccine
A new vaccine that could stop the progression of Alzheimer’s disease starts trials in humans for the first time next week, bringing hope to hundreds of thousands of Australians forced to watch a loved one slowly lose their mind.
NSW
Don't miss out on the headlines from NSW. Followed categories will be added to My News.
A new vaccine that could stop the progression of Alzheimer’s disease in its tracks starts trials in humans for the first time next week, bringing hope to hundreds of thousands of Australians forced to watch a loved one slowly lose their mind.
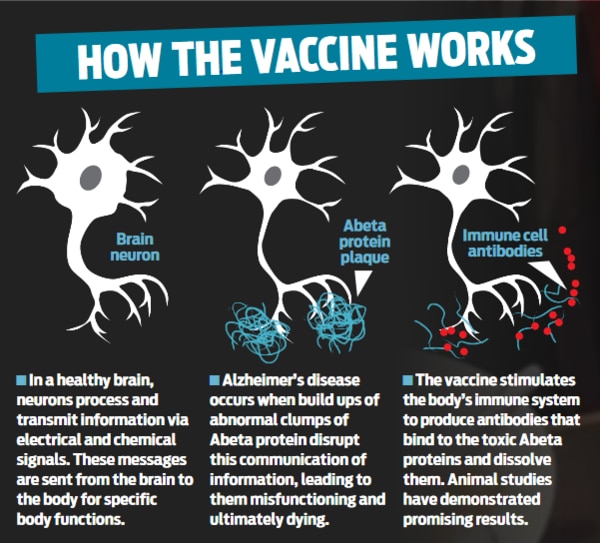
The Alzheimer’s disease vaccine stimulates the body’s immune system to produce antibodies against toxic Abeta proteins which attack the brain.
Animal studies in mice and monkeys have already demonstrated promising results, showing that the vaccine effectively reduces accumulation in the brain of clumps of Abeta protein, thereby delaying the death of nerve cells and progressive memory loss.
The phase one trial, being conducted in Adelaide, will recruit healthy male and female participants, aged 40 to 60, who will receive three doses of the recombinant protein-based vaccine over a period of approximately four months.
They will then have the level of antibodies against Abeta measured in their blood over a period of about a year to see how well the vaccine is working.

Successful outcomes from the phase one trial will pave the way for further clinical development, including testing in patients with early-stage Alzheimer’s disease, potentially offering a transformative treatment for Alzheimer’s disease patients worldwide.
The trial has arisen from a collaborative effort spanning 15 years between Australian immunologist Professor Nikolai Petrovsky and the California-based Institute for Molecular Medicine.
Prof Petrovsky said the vaccine, administered by an injection in the arm, aimed not only to slow the progression of Alzheimer’s disease but also to offer a more practical and cost-effective alternative to current treatments.
“The Alzheimer’s field has faced setbacks with unsuccessful drug candidates in late-stage trials, highlighting the urgent need for innovative therapies,” he told The Saturday Telegraph.
“Recent developments, such as the FDA (US Food and Drug Administration)-approved monoclonal antibody treatment, aducanumab, have shown promise but are costly and impractical for long-term use.”
Prof Petrovsky said the current FDA-approved treatment Leqembi, not yet available for use in Australia, was a “monoclonal antibody”.
“This is an active protein that binds to the Abeta clumps in the brain and dissolves them. The problem is that the antibody treatment breaks down relatively rapidly after it is injected into the body, so patients need to have very regular injections for it to keep working,” he said.
“By contrast, a vaccine contains a protein that looks similar to Abeta and this induces immune cells to make their own antibodies able to bind to and dissolve Abeta clumps — this effectively creates an antibody factory inside the body that can keep producing antibodies almost indefinitely, so that patients don’t have to keep getting injected all the time.”
The vaccine incorporates Prof Petrovsky’s Advax-CpG55.2 adjuvant, which acts like a car turbo charger and drives the immune cells to produce more antibodies, thereby improving the therapeutic effect.
Advax-CpG55.2 adjuvant was the first human vaccine product to be created using artificial intelligence, thereby creating a world first when it was approved for use in 2021 in Vaxine’s “SpikoGen” COVID-19 vaccine.
The development of both the Alzheimer’s vaccine and its Advax-CpG55.2 adjuvant has been supported by US government funding.
“We are extremely grateful for their tremendous support for our vaccine research programs over the last two decades,” Prof Petrovsky said.
“That is one of the key reasons we don’t see Adelaide, which is somewhat of an scientific backwater, as remaining the home for our corporate headquarters in the future, with our current plans to establish a base in the Unites States where all the cutting-edge action is.
“The problem with doing cutting-edge translational medical research in Australia is the lack of government support combined with insular and backwards-looking funding bodies.”
US Psychiatrist Dr Alexander Grinberg, who is the director of the sponsor company, Arvax Pty Ltd, said he was optimistic about the vaccine’s potential impact.
“Our preventive vaccine aims to halt or at least slow the onset of Alzheimer’s disease,” he said.
“Preventive vaccination offers a more practical and cost-effective alternative to the very expensive treatment of patients with early-stage Alzheimer’s disease.”
Got a news tip? Email weekendtele@news.com.au




