Coronavirus: Stem cell product helps Aussies as first patients injected in UK vaccine trial
Seven in ten COVID-19 patients treated with an experimental Australian stem cell product came off ventilators within ten days and most were discharged from hospital.
Coronavirus
Don't miss out on the headlines from Coronavirus. Followed categories will be added to My News.
- Australia wants wet market probe as China faces backlash
- US President contender’s brother dies of COVID-19
Seven in ten COVID-19 patients treated with an experimental Australian stem cell product came off ventilators within ten days and most were discharged from hospital.
This compares to the 9 -12 per cent of patients recovering when given standard ventilator care.
The small study which involved just 12 patients at Mt Sinai Hospital in the US was testing a stem cell product touted as being able to prevent an inflammatory response caused a cytokine storm where the body’s immune system overreacts to COVID-19 sometimes killing the patient.
In a statement issued today Australian company Mesoblast said the patients in the trial were given two intravenous infusions remestemcel-L within the first five days.
Nine of the 12 patients came off ventilator support at a median of 10 days and seven have been discharged from the hospital.
It is unclear whether the Mesoblast product was the sole cause of their recovery because patients received a variety of experimental agents prior to remestemcel-L.
Mesoblast Chief Executive Dr Silviu Itescu stated: “The remarkable clinical outcomes in these critically ill patients continue to underscore the potential benefits of remestemcel-L as an anti-inflammatory agent in cytokine release syndromes associated with high mortality, including acute graft versus host disease and COVID-19 ARDS.
The company plans to rapidly complete the randomized, placebo-controlled Phase 2/3 trial in COVID-19,” he said.
FIRST HUMAN TRIALS START IN UK
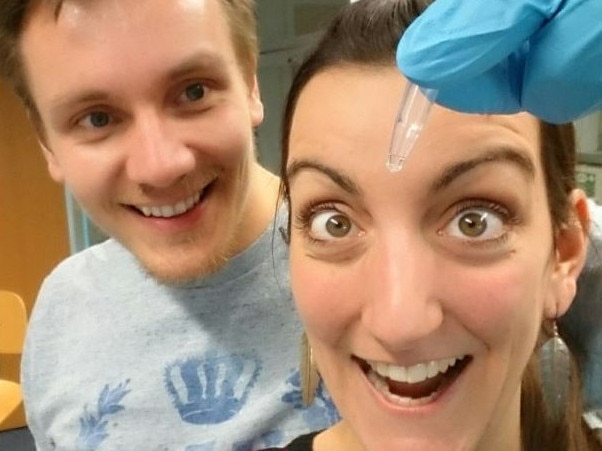
The first human trial of a coronavirus vaccine has begun in the UK.
Two volunteers at Oxford University were injected, the first of more than 800 people enlisted for the trial.
Half of the participants will reportedly receive the COVID-19 vaccine, and half a controlled vaccine which protects against meningitis, but not coronavirus.
The way the trial is designed means volunteers will not know which vaccine they are getting, though doctors will.
“I’m a scientist, so I wanted to try to support the scientific process wherever I can,” Elisa Granato, one of the two who received the injection, told the BBC:
The vaccine was developed in under three months by a team at Oxford University. Sarah Gilbert, professor of vaccinology at the Jenner Institute, led the preclinical research.
“Personally I have a high degree of confidence in this vaccine,” she said.
“Of course, we have to test it and get data from humans. We have to demonstrate it actually works and stops people getting infected with coronavirus before using the vaccine in the wider population.”
Prof Gilbert previously said she was “80 per cent confident” the vaccine would work, but now prefers not to put a figure on it, saying simply she is “very optimistic” about its chances.
JAB READY FOR PUBLIC ‘LATER THIS YEAR’
Oxford University said it had the aim of making a successful jab available to the public later this year.
Of the more than 100 research projects around the world to find a vaccine — described by the United Nations as the only route back to “normality” — seven are currently in clinical trials, according to the London School of Hygiene and Tropical Medicine.
Such trials are already underway in China and the United States and are due to begin at the end of this month in Germany, where the federal vaccine authority gave the green light on Wednesday.

The British government strongly supports Oxford University’s work, and the first human trials started on Thursday (local time), British Health Minister Matt Hancock said.
He hailed the “promising development”, pointing out that it would normally take “years” to reach such a stage of vaccine development.
In its first phase, half of the volunteers will receive the potential vaccine against COVID-19, the other half a control vaccine to test its safety and efficacy.
The volunteers are aged between 18 and 55, are in good health, have not tested positive for COVID-19 and are not pregnant or breastfeeding.
Ten participants will receive two doses of the experimental vaccine, four weeks apart.
Professor Sarah Gilbert’s team hopes for an 80 per cent success rate, and plans to produce one million doses by September, with the aim of making it widely available by the autumn if successful.
But the teams carrying out this research say on their website that this timetable is “highly ambitious” and could change.
The British government’s chief medical officer Professor Chris Whitty acknowledged on Wednesday that the likelihood of getting a vaccine within the year was “incredibly small”.
“If people are hoping it’s suddenly going to move from where we are in lockdown to where suddenly into everything is gone, that is a wholly unrealistic expectation,” he warned.
FINANCIAL GAMBLE
The strategy of not waiting for each step to be completed before launching production is a financial “gamble”, according to Nicola Stonehouse, professor of molecular virology at the University of Leeds.
But the current crisis makes it a necessary gamble, she said. The Oxford vaccine is based on a chimpanzee adenovirus, which is modified to produce proteins in human cells that are also produced by COVID-19.
It is hoped the vaccine will teach the body’s immune system to then recognise the protein and help stop the coronavirus from entering human cells.
The adenovirus vaccine is known to develop a strong immune response with a single dose and is not a replicating virus, so cannot cause infection, making it safer for children, the elderly and patients with underlying diseases such as diabetes.
The government, under fire in the media over its handling of the crisis, set up a task force last weekend to co-ordinate research efforts and to develop capability to mass-produce a vaccine as soon as it is available, wherever it comes from.
It is also supporting research at Imperial College London, which hopes to start clinical trials in June.
Their research focuses on a vaccine exploiting a different principle, using RNA, the messenger molecules that build proteins in the cells, to stimulate the immune system.
Finding a vaccine is the only possible way to bring the world back to “normality”, UN Secretary-General Antonio Guterres warned last week, calling for an acceleration of projects.
The UN on Monday adopted a resolution calling for “equitable, effective and rapid” access to a possible vaccine.
