Questions over fatal hospital drug given to Sydney man Lucas Peyret
Lucas Peyret was a fit 21-year-old who was admitted to hospital with a routine case of appendicitis. He never came home. Now his devastated mother wants answers about a controversial drug he was given.
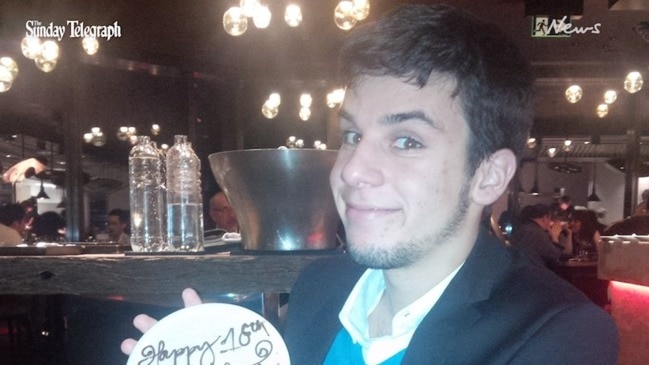
NSW
Don't miss out on the headlines from NSW. Followed categories will be added to My News.
Lucas Peyret was a fit 21-year-old who was admitted to hospital with a routine case of appendicitis — and never came home.
His mother Katya Denomme wants to know how a young man with a common condition can simply die in a NSW hospital without explanation.
Her questions have been met with conflicting accounts and, she alleges, “secrecy”.
The Sunday Telegraph can reveal a drug used on Lucas minutes before he went into cardiac arrest has been implicated in hundreds of adverse events including cardiac arrest and “life-threatening anaphylactic reaction”.
The drug, sugammadex, has been in increasing use in NSW hospitals in recent years because it allows patients to be moved quicker out of operating theatres or, as one NSW Health report puts it: “Sugammadex use permitted ‘increased throughput’ in theatres”.

“I want someone to say sorry and I want the truth. What happened that night was unacceptable and I don’t want my son’s death to be for nothing,” Ms Denomme said.
“I think they know the truth but they are trying to protect themselves.”
Both the Prince of Wales hospital’s investigation and the autopsy report to the State Coroner have found “the cause of death is unascertained”.
‘Kids will look at her funny, but she is just like them’
App to alert online users about vaccination ‘fake news’
Lucas and his mother went to Prince of Wales Hospital emergency department on Sunday May 26 after Lucas woke in pain that morning. After an examination in the emergency department, he was diagnosed with appendicitis at 2.46pm.
Lucas was admitted and examined by the surgical registrar at 6.29pm. Both he and his mother were told the operation would not go ahead that night but in the morning when a full complement of staff was on.
Later that evening Lucas sent a message to his mother saying they were coming to take him to surgery. She asked if his condition had deteriorated and he replied it had not and that he was not sure why he was having surgery that night.
He underwent surgery at 8.30pm.

“I got a call at 11.30pm and they told me he had the operation but was in a critical state,” Mr Denomme said.
According to the NSW Health report into his death, obtained by The Sunday Telegraph, the surgery was “uneventful and uncomplicated” but when the anaesthetic registrar administered 400mg of sugammadex to reverse the neuromuscular block drugs, his oxygen saturation levels plummeted and he suffered a “hypoxic cardiac arrest”.
On June 3 it was confirmed he had “irreversible brain damage” and his life support was switched off on June 5.
“He was so young and so special. He had just started a new job and he had a very promising future. I only had him,” Ms Denomme said.
The internal investigation into his death fails to identify the cause of Lucas’s death, a point Ms Denomme has found frustrating.
“It is all very blurry but he must have had a reaction. The last meeting with the hospital was appalling,” she said.
Sugamaddex has been linked to hundreds of adverse events including cardiac arrest and anaphylaxis (severe allergic reaction) in Australia and around the world.
The drug reverses neuromuscular blocks used in anaesthesia. Lucas had been given rocuronium, a drug that also has a risk of anaphylaxis but, within minutes of being administered sugammadex, Lucas went into cardiac arrest.

The hospital report notes the anaesthetic registrar gave Lucas sugammadex at 9.42pm. He then had his breathing tube removed one minute later. At 9.44pm he started wheezing and his oxygen rapidly plummeted to 20 per cent and he went blue. A code blue was called at 9.45pm and a nurse began cardiac compressions as he went into cardiac arrest. Lucas was given adrenaline as it was thought he was suffering a severe allergic reaction, or anaphylaxis. He remained in arrest for 28 minutes and suffered severe brain damage.
When Ms Denomme arrived, her only son was on life support.
“They were trying to make out they did him a favour because he was in pain but I think they rushed things because they were booked out on Monday and he’s young and healthy,” she said.
“They then said the cardiac arrest went on for so long, there might be brain damage,” she said.
A scan on June 3 showed irreversible brain damage and the life support was turned off on June 5, with Lucas’s heartbroken mother at his side.
The subsequent report into his death concluded they were “unable to identify a root cause for this incident”.
“The report is appalling. It is so outrageous, how can they take someone’s life so lightly? I am furious about the way they have treated Lucas’s life,” Ms Denomme said.
The report does say there “may have been a rare and unpredictable reaction to anaesthetic drugs” and that another cause may be “bronchospasm … which led to the development of pulmonary oedema” (fluid in the lungs).


The preliminary autopsy report also concluded “the cause of death is unascertained”.
But sugammadex has been linked to hundreds of cases of bronchospasm, pulmonary oedema and cardiac arrest in Australia and overseas.
According to Australia’s Therapeutic Goods Administration database of adverse events notifications, there have been 133 adverse events since 2008 when sugammadex was first made available. The adverse events include 61 cases of anaphylaxis, 13 cardiac arrests, 4, bronchospasms and 3 pulmonary oedema. One death was added to the database in the last week.
Although the hospital investigation did not admit to sugammadex, in a statement to The Sunday Telegraph, the TGA confirmed the death added to the database was “a 21-year-old male undergoing emergency surgery at an unidentified hospital, who experienced anaphylaxis and cardiac arrest, which was tragically fatal.”
“That’s my son,” a shocked Ms Denomme said.
The TGA statement also said: “Sugammadex, rocuronium, and vecuronium are known to be associated with hypersensitivity reactions, including anaphylactic shock. The Product Information for sugammadex includes a precaution stating that clinicians should be prepared for the possibility of drug hypersensitivity reactions, including anaphylactic reactions, and take the necessary precautions.”
In Japan, 284 cases of sugammadex-induced anaphylaxis were reported between April 2010 and June 2017.
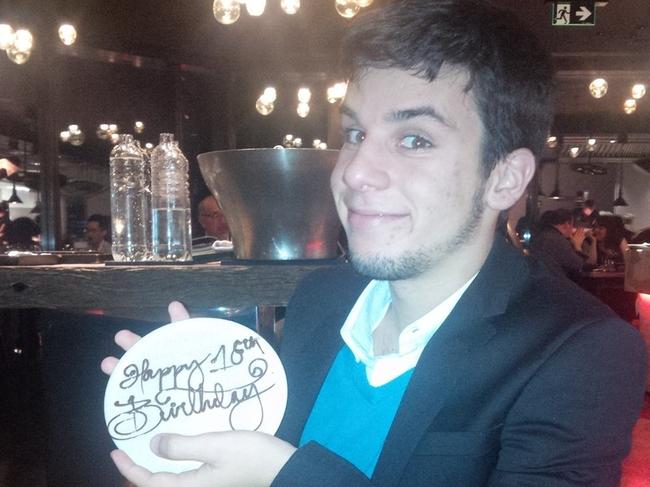
According to data from the Japanese Society of Anaesthesiologists the incidence of sugammadex-induced anaphylaxis was calculated to be approximately 29 for every 1 million administrations.
Other cases of “bronchospasm and hypoxia” have been reported in the USA, Israel, Korea and Turkey.
USA-based anaesthetist Dr David Corda has examined the adverse events of sugammadex and said: “It is widely known that Sugammadex like any other drug can cause hypersensitivity reactions. In fact, this was the concern of the Food and Drug Administration which delayed approval for its use in the United States for about 7 years after the drug was introduced into practice in Europe in 2008. Hypersensitivity reactions tend to manifest with more mild symptoms such as sneezing, nausea and rash. In a much lower incidence, Sugammadex has been linked to anaphylaxis.”
Dr Corda said that reactions tend to be dose-dependent and questioned why Lucas had been given 400mg when he only weighed around 60kg.
“In this case the sugammadex dose was 6.25mg/kg. That is an unusual dose and arguably a large one. It is known that the adverse reactions to sugammadex are dose related, ie more reaction with larger doses. It is not apparent why a large dose was used,” Dr Corda said.
Dr Corda also noted the drug safety information for Bridion, the drug’s commercial name, states “potentially serious hypersensitivity reactions, including anaphylaxis, have occurred in patients treated with BRIDION. In a clinical study, anaphylaxis occurred in 0.3% (1 in 333) of healthy volunteers treated with BRIDION,” a rate he called “eye-watering”.
“The patient’s symptoms of wheezing and cardiovascular collapse certainly make an anaphylactic reaction high on the differential diagnosis. The timing of the event in relation to administration of sugammadex would place that as a potential cause,” Dr Corda said.

The use of sugammadex has soared in recent years, so much so that the NSW Therapeutic Advisory Group, NSWTAG, investigated the increase in NSW hospitals in 2018 and asked NSW hospitals if they had “identified an increase in usage of sugammadex in the past 1 to 2 years?”
South East Sydney Local Health District, where Prince of Wales is based, reported they had (as did Canterbury, Liverpool, St Vincent’s and several others).
But the SESLHD told the NSWTAG they had “a greater than ten-fold increase in use over 3 years”. Sugamaddex had been restricted up until 2015 for “urgent reversal” of neuromuscular blockers only.
While Liverpool Hospital cited “extensive marketing by the company as a reason for their increased use of sugammadex”, the directors of anaesthetics at the SESLHD found the “formulary restriction (urgent reversal only) was misaligned with current clinical practice” and in 2016 had the restriction lifted for use in “reversal of rocuronium/vercuronium induced neuromuscular blockade”, which means it is now the drug of choice to reverse rocuronium — which Lucas had been given.
The SESLHD also negotiated with the pharmaceutical company, Merck Sharp & Dohme for a lower price for the drug.
“We were able to negotiate a consistent and satisfactory price for the LHD with the manufacturer based on increased usage,” the SESLHD told the NSWTAG.
Tamworth hospital told the NSWTAG that the use of sugammadex “had escalated rapidly over that time due to ‘perceptions’ that sugammadex use permitted ‘increased throughput’ in theatres, with fewer adverse effects”.
Ms Denomme took her son’s ashes to her native France and recently returned to Sydney to pursue her legal options.
“Sydney is not home any more, once my son’s case is clarified or solved, I will leave this city definitively,” she said.
Originally published as Questions over fatal hospital drug given to Sydney man Lucas Peyret


