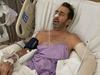
Mark Briggs’s wife says specialist has told her AstraZeneca was responsible
The Rockhampton father-of-four has been hospitalised for more than nine weeks. An exhaustive battery of tests had failed to provide a diagnosis – until now.
Rockhampton
Don't miss out on the headlines from Rockhampton. Followed categories will be added to My News.
Mark Briggs’s life-threatening symptoms have been caused by a reaction to the AstraZeneca vaccine, a Brisbane specialist has told his wife Sam.
Mr Briggs, a 55-year-old father-of-four, has spent more than nine weeks in hospitals in Rockhampton and Brisbane.
An exhaustive battery of tests had failed to provide a diagnosis – until now.
Mrs Briggs said the specialist heading up her husband’s case told her he was “100 per cent sure” it was a response to the AstraZeneca vaccine.
An MRI done this week showed Mr Briggs had myocarditis, an inflammation of the heart.
A neurologist is investigating whether Mr Briggs may also have Guillain-Barre Syndrome, rare events of which have been reported following vaccination with AstraZeneca.
Mrs Briggs said the specialist was going to report it to the Therapeutic Goods Administration (TGA).
“He believes the AstraZeneca (vaccine) has caused heart failure and the heart failure has caused all of these other things,” she said.
“He said he was going to write a report that it’s a reaction response to the vaccine.
“He said it probably won’t do anything other than become a statistic but we don’t care, we just want it to be written up so that if somebody else goes through it then at least there’s a case for them to look at.
“By the sound of it, he’s done a heap of studies of his own. Apparently, his back interest is vaccines and particularly vaccines made from DNA.
“He told me he’s found 13 cases of this, what Mark’s got, in the UK.”
Mrs Briggs said two other specialists on her husband’s case supported the diagnosis.
The Morning Bulletin attempted to contact the specialist, but The Wesley Hospital said it was unable to comment on the case for privacy and confidentiality reasons.
Mr Briggs’ mother Ienis, who is in her 70s, had her first AstraZeneca jab three days after Mark and had no side effects.
The family suspected the vaccination after Mr Briggs, who was fit and well and had no underlying medical conditions, started showing symptoms just over a week after having his first dose on June 15.
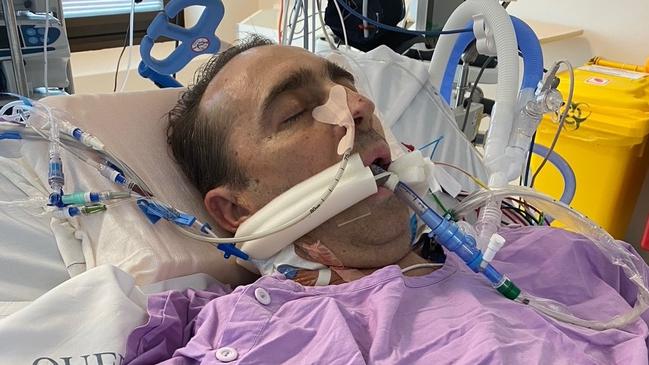
He was hospitalised on June 24 and within days was critically ill.
At his worst, Mr Briggs was intubated, had myopericarditis, severe pneumonia and sepsis. His liver was severely inflamed, his kidneys were shutting down and he had 12 litres of fluid on his body.
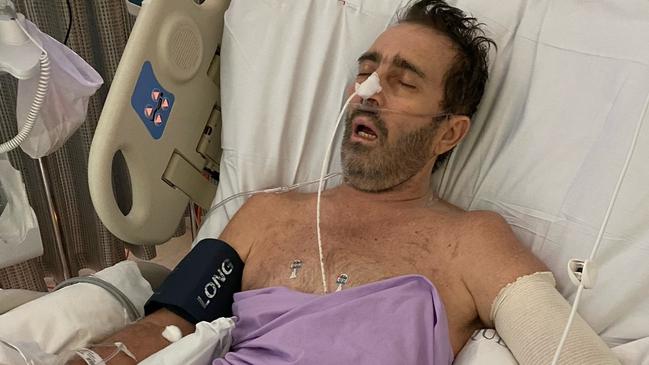
Three times he almost lost his life, but he fought back and is now in The Wesley Hospital in Brisbane where he is showing some improvement.
The Briggs family had previously been told his case had been referred to the TGA for investigation, but it has not yet been included in its Covid-19 vaccine weekly safety report.
Discharge papers from Mr Briggs’s first stint in Rockhampton Hospital list under the consultation notes: “Covid-19 Adverse Event Following Immunisation reporting completed”.
Mr Briggs’s reaction to the vaccine is rare, with the TGA’s latest weekly safety report saying that up to August 22, 2021, more than 17.1 million doses of Covid vaccines had been administered.
The TGA has found that seven reports of deaths were linked to immunisation from 476 reports received and reviewed. These deaths occurred after the first dose of the AstraZeneca vaccine – six were thrombosis with thrombocytopenia syndrome cases and one a case of immune thrombocytopenia (ITP).
Total Australian reports assessed as TTS following the AstraZeneca vaccine to 116 cases (67 confirmed, 49 probable) from about 8.8 million vaccine doses.

According to the report, a warning statement about myocarditis and pericarditis (inflammation of the membrane around the heart) have been included in the Consumer Medicine Information and Product Information for Pfizer - but those conditions do not appear as side effects of the AstraZeneca.
However, official government health data from the United Kingdom shows that up to and including August 18, 2021, there were 95 reports of myocarditis and 150 reports of pericarditis following the AstraZeneca vaccination.
For AstraZeneca, the overall reporting rate for myocarditis (including viral myocarditis and infectious myocarditis) is 2.0 per million doses and for pericarditis (including viral pericarditis) is 3.1 per million doses in the UK.
It is estimated that in the UK there are about six new cases of myocarditis per 100,000 patients per year and about 10 new cases of pericarditis per 100,000 patients per year.
The Australian Technical Advisory Group on Immunisation stresses that “vaccination is a key public health intervention to prevent infection, transmission and severe disease”, and it continues to recommend Covid vaccination for all adults, and specific high-risk adolescents.

Mrs Briggs said it was a huge relief to finally have a diagnosis.
“We’re just really grateful because now we can treat what’s going on with him.
“We just want Mark to get better.”
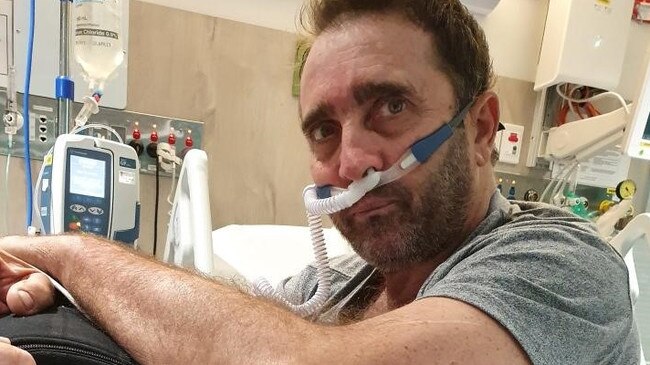
Mr Briggs is out of intensive care and in a ward. He is off all breathing support and is eating and walking with assistance.
He still has DVTs in his legs and left arm and is on medication for the heart failure and three medications to control his fluctuating blood pressure.
“This is definitely the best he’s been for a long time,” Mrs Briggs said.
“He seems to be on the improve but we’re still going to have bad days.
“They say that there will be days when he goes forward and then days where you have a little bit of a hump, which we’re expecting.”