Johnson & Johnson: Class action filed against global pharma giant over ‘ineffective medicines’
A Brisbane law firm has filed a class action against Johnson & Johnson, with explosive claims it has knowingly been selling ineffective medicines. FULL LIST OF PRODUCTS

QLD News
Don't miss out on the headlines from QLD News. Followed categories will be added to My News.
One of the biggest class actions in Australia has been filed in the Federal Court against pharmaceutical giant Johnson & Johnson, with explosive claims they have knowingly been selling trusted medicines to Australians that do not work.
And the cold and flu products are so common in households that tens of millions of people in Queensland and across the country could be eligible for compensation which would make it the biggest class action in the country in terms of class members.
SCROLL DOWN TO SEE THE FULL LIST OF AFFECTED PRODUCTS
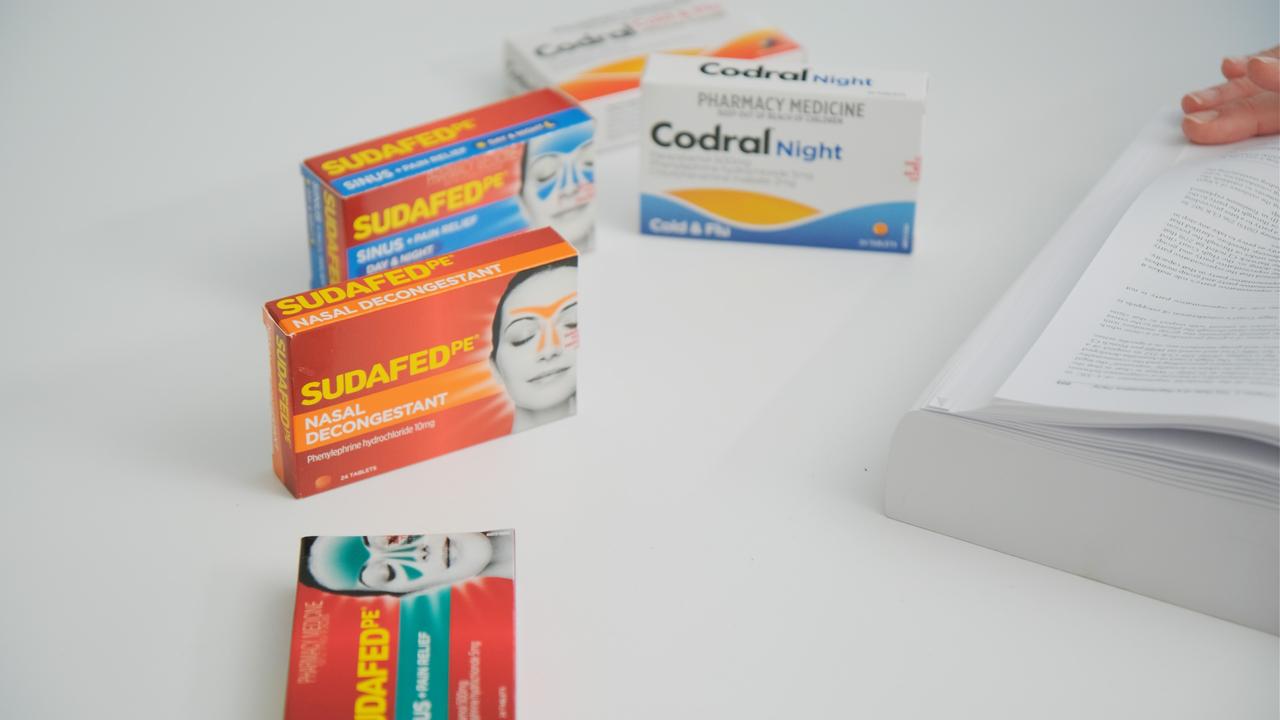
Codral Cold & Flu, Sudafed PE and Benadryl are just three of many products which contain phenylephrine, a drug the company has marketed and sold as a decongestant for years.
However, a growing body of evidence has the Food and Drugs Administration (FDA) in the United States considering an outright ban because it believes the drugs when taken orally, don’t work.
JGA Saddler lawyers, who specialise in large and complex litigation with a focus on class action, are calling on anyone who has bought any of the products in the last 18 years to
register for the class action at coldandfluclassaction.com.au.

JGA Saddler director Rebecca Jancauskas said it was a case of Johnson & Johnson putting profit before people.
“Customers should be able to confidently buy medicines that work as advertised and when they don’t, the company involved should be held accountable,” Ms Jancauskas said.
“These products have been dominating the market so we know a lot of people have purchased them in good faith,” she said.

“Johnson&Johnson has manufactured and marketed a medication that decades of evidence have shown doesn’t work as claimed, relying on outdated, fallible studies to sell the Australian public products that don’t do what they claim on the packet.
“Australians have trusted these products to work as advertised and wouldn’t have bought them if they realised they were ineffective at treating congestion,” the lawyer said.
When the decongestant pseudoephedrine, which is effective when taken orally, was moved behind the counter in 2006 over concerns it was being used in the manufacture of illicit drugs, phenylephrine was quickly marketed as a substitute.
Brisbane ear, nose, and throat specialist Dr Jo-Lyn McKenzie said the marketing of phenylephrine as an oral decongestant didn’t live up to the reality.
“It’s unconscionable and deeply unethical for corporations to sell healthcare products while knowing they don’t work. This practice erodes trust in a space that depends on consumer confidence,” Dr McKenzie said.

“When patients feel misled or duped, they may begin to question the validity of evidence-based treatments that are genuinely effective. This situation serves as a reminder for Australian consumers to be cautious and informed. Rather than relying on direct-to-consumer advertising, take the time to have meaningful conversations with health professionals who can provide evidence-based recommendations,” the specialist said.

This case is backed by global litigation funder Omni Bridgeway. Omni Bridgeway’s
Investment Manager Niall Watson-Dunne said it was important companies are held
to account when they breach the guarantees owed to consumers.
“For around 19 years, Australians have been sold cold and flu products to relieve
their symptoms, despite studies and scientific evidence showing their key ingredient
phenylephrine is ineffective when taken orally,” Mr Watson-Dunne said. “Australians
deserve better.”




