2025 has plenty to offer in the health sphere – and Australians are at the forefront of treatments
Australian specialists, scientists and researchers are at the forefront of treatments that could change the lives of people all over the world. Here’s what 2025 has to offer.

Health
Don't miss out on the headlines from Health. Followed categories will be added to My News.
From an oral treatment for hair loss to personalised medicine and Australia’s first
cancer screening program in decades, 2025 has plenty to offer in the health sphere.
Australian specialists, scientists and researchers are at the forefront of treatments
that could change the lives of people all over the world.
PILL FOR HAIR LOSS

A hair loss treatment pioneered by Melbourne Professor Rodney Sinclair is gathering momentum around the world.
Minoxidil in liquid form is a common hair loss treatment but has had limited success, but the pill form is helping patients regrow hair.
A topical form of Minoxidil has been approved in Australia for many years and can be found on the shelves of most pharmacies.
But ironically the oral version, when used as a blood pressure medication, has not been
widely used because of the reported side effects of increases in body hair.
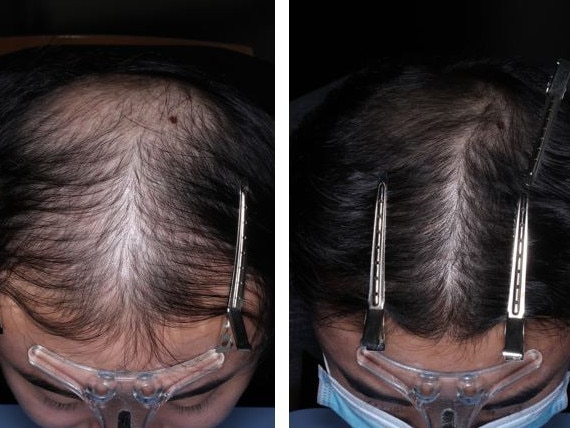
Professor Sinclair has described the treatment as a “game changer”.
He suggested “in 10- or 20-years’ time, it’s going to be a little bit like crooked teeth and
braces”.
“So when I was a kid, lots of people had crooked teeth because braces were
phenomenally expensive. Nowadays there are so many alternatives and, relatively
speaking, it’s not nearly as expensive.
“I think it be the same with hair loss.”
Low-dose oral Minoxidil (LDOM) is not approved anywhere for the treatment of hair loss but is used off label in Australia and other parts of the world.
Professor Sinclair has just completed a phase I study of LDOM. The next phase is planned for early 2025 and needs about 250 participants. If all goes well, the medication could be approved and available around the middle of 2026.
NEW ALTERNATIVE TO STATINS FOR LOWERING CHOLESTEROL

Specialists and patients are hopeful a new non-statin, cholesterol-lowering drug will
go to the Therapeutic Goods Administration (TGA) for approval in Australia in 2025.
The drug, known as bempedoic acid, has already been approved for use in the US
and Europe, and the US-based company that makes the drug has recently applied
for approval in Canada, Israel and Japan.
The company has said it intends to make an application to the TGA for approval to
supply the drug to Australians but is yet to commit to a date.
An international clinical trial has shown bempedoic acid can reduce LDL cholesterol
by up to 25 per cent. Cardiologist Professor Stephen Nicholls, director of the
Victorian Heart Hospital at Monash Health and Monash University’s Victorian Heart
Institute, co-led this trial, which included more than 300 Australian participants.
And while he describes the drug as a gamechanger for statin-intolerant patients, he
believes statins will remain the first-line treatment for high cholesterol.
It is estimated that some 2.5 million Australians take statins to lower their cholesterol.
Professor Nicholls said about 20 per cent of those had issues tolerating the treatment
and about 50 per cent of high-risk patients didn’t get their cholesterol down to target
levels so they needed other options.
He knows the drug works but said there were challenges in getting TGA approval.
“One of the challenges is the company that developed bempedoic acid is a
reasonably small company, and so Australia tends to fall a little down the list. We’re
hoping they or perhaps someone will partner with them to see the opportunity here.”
WEIGHT-LOSS MEDICATIONS
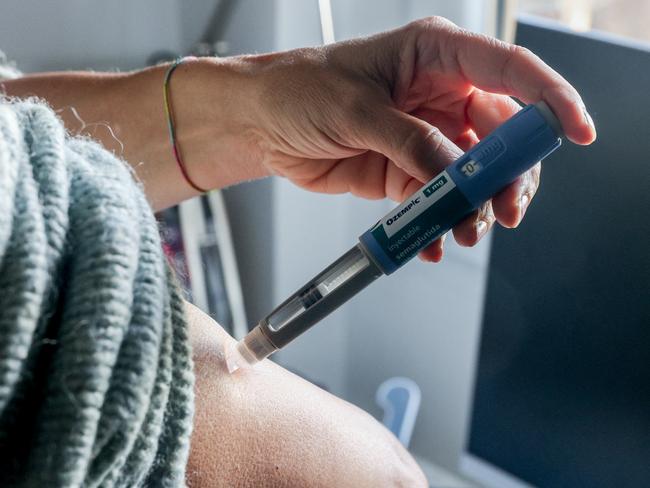
Self-injectable medications such as semaglutide and tirzepatide, originally developed to treat type 2 diabetes, have become some of the most discussed weight loss treatments of the decade.
Globally, the availability of semaglutide (marketed as Ozempic) has been limited, partly due to its increasing off-label use for weight loss.
In Australia, both semaglutide and tirzepatide have been approved by the TGA; however, they are only subsidised under the Pharmaceutical Benefits Scheme (PBS) for specific patients with type 2 diabetes.
Many people have forked out hundreds of dollars a month to buy the medication privately.
But weight loss and diabetes seem to be just the tip of the iceberg when it comes to
health benefits. Research published on an almost weekly basis this year is
uncovering some stunning results for the drugs, and not all the effects can be
attributed to weight loss or better diabetes control alone. More recently, the drugs
have been linked to improvements in cardiovascular and kidney health, Alzheimer’s
incidence, knee osteoarthritis and even asthma.
Monash University’s Professor Stephen Nicholl said Australian regulators would be facing growing pressure to provide subsidies through the PBS, beyond existing approvals, due to the clinical trials of these therapies and the benefits that they are demonstrating.
PERSONALISED MEDICINE GETS A STEP CLOSER
Genomics Australia – a new body with a focus on turning significant breakthroughs
in genomic research into the everyday care of patients – opens for business on July
1, 2025.
Genomic medicine holds the promise of delivering more personalised, precise, and
effective therapies, ranging from early disease detection to targeted treatments for
various conditions such as cancer, genetic disorders, and rare diseases.
Establishing a dedicated organisation focused on translating research into clinical
practice can play a crucial role in bridging the gap between laboratory discoveries
and real-world patient outcomes.
NATIONAL LUNG CANCER SCREENING PROGRAM

Another national program taking flight in July 2025 is the new National Lung Cancer
Screening Program – the first national cancer screening program in Australia in about two
decades.
Australians will be eligible for the program if they show no signs or symptoms of lung
cancer and have a history of at least 30 pack-years of cigarette smoking and are still
smoking; or have a history of at least 30 pack-years of cigarette smoking and quit in
the past 10 years.
The screening program uses low dose computed tomography scans to look for lung
cancer in high-risk people without any symptoms. The aim is to screen all high-risk
people aged 50-70 years every two years.
Lung Foundation Australia CEO Mark Brooke said the initiative could save more than 500 lives each year.
“Lung cancer is a leading cause of cancer death in Australia, but if found early, more
than 65 per cent of lung cancer cases can be treated successfully,” he said.
Originally published as 2025 has plenty to offer in the health sphere – and Australians are at the forefront of treatments


