Counterfeit Ozempic-labelled pens imported into Australia
Australians have been warned about counterfeit Ozempic-labelled pens being imported into the country. See how to spot the difference.

Health
Don't miss out on the headlines from Health. Followed categories will be added to My News.
The Therapeutic Goods Administration (TGA), in collaboration with the Australian Border Force (ABF), has warned of counterfeit Ozempic-labelled pens being imported into Australia.
The TGA says that while the pens are undergoing ‘laboratory testing’, the safety and quality of the products are unknown and therefore should not be used.
These are some of the ways to detect counterfeit products: spelling errors, the instruction leaflets might not be in English, unsealed packaging, changes in medicine size, shape, or appearance.
Consumers should be warned that manufacturers of counterfeit goods are producing products that to the untrained eye may appear legitimate - you should purchase your medicines from legitimate sources.
Similar counterfeit products identified overseas have been associated with life-threatening adverse events, including hospitalisation, due to insulin being unknowingly injected instead of the expected semaglutide.

The products detected by ABF were purchased online from an overseas website.
Although consumers who hold a valid prescription can lawfully import prescription-only medicines such as Ozempic under the personal importation scheme, counterfeit products − whether bought knowingly or unknowingly − cannot be imported under any circumstances, even if a prescription is provided.
The TGA advises to always buy medicines from reputable sources and consult your healthcare provider or local registered pharmacy if you have any concerns and to exercise extreme caution when buying medicines from unknown overseas websites.
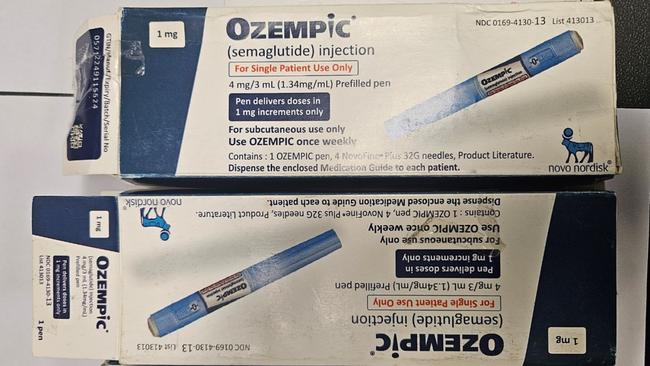
The TGA is continuing to check batch numbers of any products that may be subject to counterfeiting and is working with the ABF to help stop future shipments of counterfeit and substandard Ozempic from entering Australia.
Call 000 or get other emergency medical assistance if you or someone else has symptoms of severe low blood sugar levels after using a product labelled as Ozempic. This might include weakness, trembling, shaking, confusion and unconsciousness.
If you have any other concerns about using a product labelled as Ozempic, consult your health care practitioner.
If you suspect you have had a side effect (also known as an adverse event) to this or a similar medicine, report it to the TGA.
If possible, keep the medicine, as the TGA may request it for testing.
Call the TGA on 1800 020 653 or email info@tga.gov.au.
Originally published as Counterfeit Ozempic-labelled pens imported into Australia