WHO sees ‘potentially positive data’ in treating coronavirus
The World Health Organisation says it’s turning its focus to four or five treatments that have proven to limit the severity of coronavirus.
Coronavirus
Don't miss out on the headlines from Coronavirus. Followed categories will be added to My News.
- The antibodies that could stop spread of COVID-19
- Aussie hospitals run out of critical COVID-19 medicines
The World Health Organization says that some treatments appear to be limiting the severity or length of the COVID-19 disease and that it was focusing on learning more about four or five of the most promising ones.
“We do have some treatments that seem to be in very early studies limiting the severity or the length of the illness but we do not have anything that can kill or stop the virus,” spokeswoman Margaret Harris told a briefing.
“We do have potentially positive data coming out but we need to see more data to be 100 per cent confident that we can say this treatment over that one,” she added, saying more research was needed and planned.
Harris did not name the treatments.
VACCINES LEADING THE RACE
Patients given Remdesivir recovered in 11 days compared to 15 days for those who did not receive the treatment, a study funded by the US National Institute of Allergy and Infectious Diseases (NIAID) and pharmaceutical company Gilead found.
Only eight per cent of those using the drug died compared with 11 per cent of those given a placebo.
The results of the global trial in over 1000 patients were so promising the study was ended early so all patients could be given the drug.
However, the outcomes of a second trial of the same drug in 237 Chinese patients published in The Lancet medical journal found it did not speed recovery or reduce death rates from the virus.
However, it did find patients given Remdesivir in the first 10 days of the infection recovered quicker and spent less time on a ventilator.
“Although a 31 per cent improvement doesn’t seem like a knockout 100 per cent it is a very important proof of concept,” Dr. Anthony Fauci, NIAID director, said.
“What it has proven is that a drug can block this virus.”
The patients in the global trial were given 10 days of treatment.
Earlier this month, a clinical trial in Chicago indicated only six days of treatment may be needed meaning you could treat twice as many people with the supply already available.
Unlike the latest studies, the Chicago trial did not compare outcomes with people not given the drug.
Of the 113 people in the Chicago trial all but two COVID-19 patients appeared to respond to the drug and they were discharged from hospital within a week. Two people on the trial died.
The treatment was originally developed to treat ebola and works by blocking an enzyme many viruses use to copy themselves, if the virus can’t copy itself it can’t reproduce and survive in the human body.
A worldwide shortage of the drug means most patients with COVID-19 will be unable to access it just yet.
A landmark Australian COVID-19 trial in over 70 hospitals will not get to test the drug and to date, no Australian patient has received the treatment.
Pharmaceutical company Gilead which manufacturers Remdesivir has told News Corp it will not supply any doses of the drug to the ASCOT trial taking place in 70 hospitals here.
“In Australia we do have access to a small volume of Remdesivir under a restricted compassionate use program,” a spokesperson for Gilead said.
“This is reserved for those hospitalised patients with severe complications who are pregnant or under eighteen years of age.”
JAPAN TO FAST TRACK REMDESIVIR REVIEW
Japan will fast-track a review of Gilead Sciences Inc’s antiviral drug remdesivir so that it can hopefully be approved for domestic COVID-19 patients a week after the US firm’s filing for such approval, said Health Minister Katsunobu Kato.
The comment comes after remdesivir was granted emergency use authorisation by the US Food and Drug Administration for COVID-19 on Friday local time.
“I’ve heard that Gilead Sciences will file for approval (in Japan) within days,” Mr Kato told reporters. “I issued an instruction so that we will be ready to approve it within a week or so.” Prime Minister Shinzo Abe said he was leaning towards extending Japan’s state of emergency, due to expire on May 6, for a month as experts said coronavirus restrictions should remain in place until the number of cases falls further.
The Nikkei business daily has said although Gilead planned to distribute enough doses to cover 140,000 patients worldwide, Japan would not receive enough for all its patients in need.
No one was immediately available for comment at Gilead’s Japanese unit. Remdesivir, which previously failed as a treatment for Ebola, is being tried against COVID-19 because it is designed to disable the mechanism by which certain viruses, including the coronavirus, make copies of themselves and potentially overwhelm their host’s immune system.
THE MOST PROMISING VACCINES
There are around 119 vaccines under development to prevent COVID-19. These research teams are leading the race with human trials.
OXFORD UNIVERSITY
Oxford University’s Jenner Institute is leading the race for a COVID-19 vaccine and began human trials on over 1000 people on April 23. Next month the trial will be expanded to include another 5000 people.
While it was not the first to begin human trials, the Oxford team is closer to mass producing a vaccine because its technology had been proven safe to use in humans in the past.
Last month, six monkeys were given the Oxford vaccine and then exposed to coronavirus, 28 days later none had become ill.
The vaccine is also being tested on ferrets at the CSIRO’s animal testing laboratory in Melbourne.
“It certainly hasn’t done any harm in the animals,” Dr Rob Grenfell, the CSIRO’s Health Director of the Health and Biosecurity Business Unit, told News Corp.
Over the next two months the CSIRO will challenge vaccinated ferrets with the COVID-19 virus to see if it protects them.
It is not ethical to infect human trial subjects with COVID-19 to see if the vaccine works so instead it will have to be tested by inoculating people in a community where COVID-19 is spreading and checking whether they catch the virus naturally.
The vaccine is based on a chimpanzee adenovirus, a weakened version of a common cold virus that has been genetically changed to grow in humans.
The team hopes to be able to produce several million doses of the vaccine by September.
The Oxford team is working with pharmaceutical companies across Europe and Asia to prepare to churn out billions of doses as quickly as possible if the vaccine is approved.
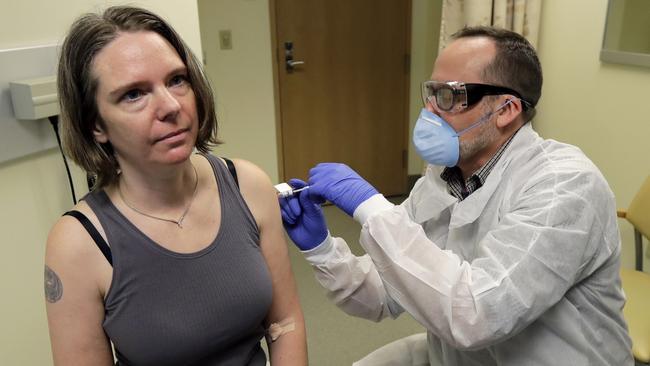
MODERNA
US biotech company vaccine Moderna was the first research team to commence human clinical trials of a COVID-19 vaccine on March 16.
Last week the company gave the 45 trial participants a second dose of the vaccine and this was a good sign because it indicated there had been no safety issues with the initial dose.
The same group will be given a third dose next month, 28 days after they received the second dose.
These trials check whether the vaccine is safe to use in humans and the next stage of the trials will determine whether it works to prevent people being infected with the virus.
The plan is to expand the trial to enrol 600 healthy adults and older adults in two cohorts.
Larger phase three trials in thousands of people are scheduled to begin in September.
The company has said under emergency use, a vaccine could be available to some people, possibly including healthcare professionals by September 2020.
The Moderna vaccine is an mRNA vaccine which the genetic information from the virus to make our own cells make a protein that raises an antibody response to the virus.

INNOVIO
US company Innovio began human testing of its DNA vaccine for COVID-19 on April 6 and has already reported promising results.
This week it announced vaccine recipients demonstrated strong antibody and T cell immune responses after two or three doses of the vaccine.
The vaccine did not appear to have any safety issues and was well tolerated by people taking part in the trial.
One hundred per cent of people developed antibodies in their blood after three doses given at 0, 4 and 12 weeks, 84 per cent developed antibodies after two doses.
South Korea’s International Vaccine Institute has received funding to start a second human clinical trial of the vaccine.
The Innovio DNA vaccine candidate works by injecting a specifically engineered genetic structure into a patient to prompt their cells to produce a targeted antibody to fight off a specific infection.
The US trial began in 40 healthy adults and then expanded to older adults.
CHINA
Chinese company Sinovac Biotech has started human clinical trials of its COVID-19 vaccine in 144 participants to test if it is safe in humans.
It is using a chemically inactivated form of the SARS-CoV-2- virus that causes COVID-19 to build its vaccine.
Two weeks ago it reported its vaccine was effective in Rhesus monkeys.
Two different doses of the vaccine were given to eight Rhesus monkeys and three weeks later the animals were challenged with the SARS-CoV-2, the virus that causes COVID-19.
The virus was introduced into the monkeys’ lungs through tubes down their tracheas, and none developed a full-blown infection.
Some of the animals that received a lower dose of the vaccine showed some signs of the virus but none developed a full blown case.
The company is planning to begin phase 2 trials in 1000 people in mid-May to test if the vaccine protects humans from the virus.
Another Chinese team, Sinopharm commenced human clinical trials of a COVID-19 vaccine using an inactivated form of the SARS-COV-2 virus on April 23
Ninety six volunteers across three age groups were injected and the company said the vaccine appeared safe with the trial participants still under observation.
It has produced over 50,000 doses for the initial clinical trials and plans to make 100 million doses a year.
China is also testing another vaccine using the adenovirus which can cause the common cold. It was developed by Institute of Military Medicine.
GERMAN
German pharmaceutical company BioNTech has began testing a potential vaccine for COVID-19 on human volunteers on April 23.
The company which is working with the US pharmaceutical giant Pfizer gav doses of its vaccine to 12 participants in a clinical trial in Germany.
In a statement the company said in the next step will involve increasing the dose of the vaccine in a trial involving about 200 participants aged 18 to 55.

UNIVERSITY QUEENSLAND
The University of Queensland is hoping to begin human trials of its COVID-19 vaccine by July and is hoping to start large-scale production by September.
The team announced this week its molecular clamp vaccine had worked in mice which produced a strong immune response when injected with the vaccine at Melbourne’s Peter Doherty Institute.
When blood from the mice was tested on the SARS-CoV-2 virus in a test tube the virus was killed.
The strength of the antibody response to the vaccine in mice much higher than that achieved in samples from patients who had recovered from the virus.
The vaccine will now be tested in live ferrets and hamsters in the Netherlands in Dutch company Viroclinics biosecurity laboratory.
The CSIRO’s biologies production facility in Melbourne has been producing small samples of the vaccine for testing.
And the university team has been given access to key adjuvant technology from CSL/Seqirus, Dynavax and GSK to help make the vaccine.

PERTH
Perth-based Linear Clinical Research is preparing to lunch human trial of COVID-19 vaccine developed by Chinese company Clover Biopharmaceuticals Australia.
It announced this week it would begin to recruit healthy Australian adults for the trial within next two months.
Its S-Trimer vaccine targets a protein that the SARS-COV-2 virus needs to enter host cells.
MONASH UNIVERSITY
Monash University in Melbourne has identified three COVID-19 vaccine candidates which will be tested on mice within weeks.
The university is using fast-paced mRNA technology to develop vaccines that could protect against the virus.
It uses genetic information from the virus to prompt our cells to make a protein that raises antibodies to the virus.
Professor Colin Pouton said the vaccines will be injected into mice to see if they raise antibodies to the virus.
If that works the blood from the mice will be tested on samples of the virus in test tubes at the Doherty Institute to see if they prevent growth of the virus in cell culture.
It will take six weeks to test the mice because they will need to be given two doses of the vaccine., it will take another two weeks to get results from the Doherty Institute.
“We are hoping to be able to choose the vaccine that produces the strongest antibody response,” he told News Corp.
If the tests are successful the university will need funding to conduct human clinical trials of the vaccines.
Originally published as WHO sees ‘potentially positive data’ in treating coronavirus
