Allergy warning over Pfizer COVID vaccine
People with “a history of allergic reactions” must now avoid the Pfizer COVID vaccine after two health workers suffered symptoms after getting the shot.
Coronavirus
Don't miss out on the headlines from Coronavirus. Followed categories will be added to My News.
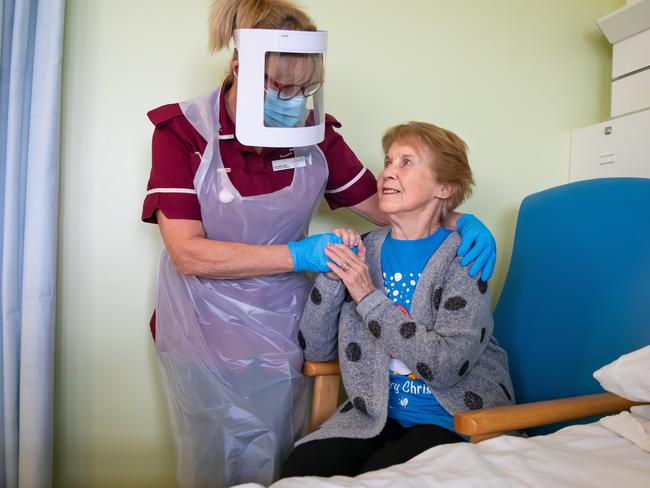
People who suffer severe allergic reactions have been advised by UK regulators not to take the Pfizer COVID-19 vaccine after two British nurses who received the jab suffered allergic reactions.
The nurses had a history of allergic reactions and carried EpiPens to combat them and are said to be recovering well after the incident.
The UK’s medical watchdog the Medicines and Healthcare Products Regulatory Agency (MHRA) issued precautionary advice that anyone with a history of significant allergic reactions to medicines, foods or vaccines should not receive the vaccine.
Reports suggest the nurses suffered skin rash, breathlessness and drop in blood pressure after receiving the vaccine but did not suffer anaphylaxis which can be fatal.
Australia’s medicines regulator has not yet decided whether to advise people with allergies to avoid the Pfizer COVID-19 vaccine even though UK authorities have done so.
The Therapeutic Goods Administration (TGA) told News Corp “it is too early to speculate which warnings may be included” for the vaccine.
The TGA said it “continues to receive and assess ongoing vaccine efficacy and safety data from Pfizer, and other vaccine developers, as part of the Australian provisional registration assessment process”.
“We are also working closely with international regulators, including in the United Kingdom, to ascertain real-world safety information as it comes to hand. All available safety information will be considered as part of our assessment process,” the Administration said.
Once a vaccine is approved in Australia it will have Product Information and Consumer Medicine Information which will describe potential side effects in addition to special warnings and precautions.
Experts are urging the public not to be alarmed by the allergic reactions and to have confidence in the vaccination.
To put it into perspective they say one in a thousand people in the UK have died from coronavirus while two people, out of thousands vaccinated yesterday, had an allergic reaction which they recovered from.
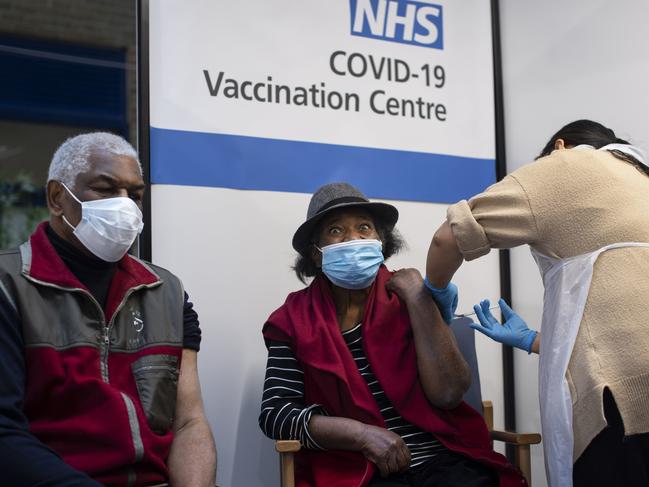
The incident occurred as Canada’s medicines regulatory agency approved the use of Pfizer’s vaccine in that country.
The US regulator the Food and Drug Administration is due to consider whether to approve the vaccine today.
Overnight the United Arab Emirates approved the use of a Chinese made vaccine produced by Sinopharm after reviewing preliminary trial data they said showed it was 86 per cent effective.
These trial results have not been published and there is uncertainty about how the effectiveness was measured.
The Sinopharm vaccine relies on an inactivated version of the virus that causes Covid-19 and there are concerns it might trigger a more severe reaction.

SIX PEOPLE DIED DURING PFIZER TRIAL
Six people that took part in the Pfizer-BioNtech COVID-19 vaccine clinical trial died – including four who had received a placebo shot but the vaccine was unlikely to be the cause of their death.
The six deaths out of about 44,000 trial participants were detailed in a 53-page briefing the pharmaceutical companies provided to the US Food and Drug Administration (FDA) yesterday.
The FDA is expected to decide within days whether to approve the vaccine for use in that country.
The pharmaceutical company said the deaths were not due to the vaccine and were the types of events that happened in the general community to people in this age group.
“All deaths represent events that occur in the general population of the age group where they occurred, at a similar rate,” the report said.
One of the people who received the vaccine and died was older and experienced thickening of the arteries that cut off blood supply to their organs. This person died three days after receiving the first dose of the vaccine.
Another person in the older age group who received the vaccine suffered a cardiac arrest 60 days after receiving the vaccine and died three days later.
The briefing paper also revealed the vaccine provides strong protection against COVID-19 after just one shot.
Ten days after the first dose of the vaccine 52 per cent of people were protected from COVID-19, after two doses the vaccine was 95 per cent effective the papers reveal.
The coronavirus vaccine made by Pfizer and BioNTech is being rolled out in Britain this week.
Australia has 10 million doses on order but our regulator is unlikely to approve it until January next year.
The briefing materials show the vaccine works well in both men and women and had similar efficacy rates in white, Black and Latino people.
Obese people are at greater risk from COVID-19 and the clinical trial showed it worked well in them as well as the elderly.
A large number of people felt unwell after receiving the vaccine with half developing fatigue and headaches, one in three felt chills and muscle pain.
welve cases of severe and life-threatening appendicitis occurred in the trial – including eight people who received the Pfizer vaccine and four on the placebo – but they were “not considered related to study intervention”
The cases were “not greater than expected based on background rates estimated in a US Electronic Health Records database”, the analysis said.

Sixty four people in the trial developed swollen lymph nodes in the arm and upper neck region within two to four days after the injection. Of these, 58 received the Pfizer vaccine and six received the placebo.
Most of those reporting this reaction (54) were younger and female.
“The average duration of these events was approximately 10 days, with 11 events ongoing at the time of the data cut-off,” the report said.
Younger people were more likely to suffer side effects from the vaccine than older people and the number of people experiencing side effects rose after they received the second dose of the vaccine.
This table shows the difference and reports how many experienced side effects after the first dose (the first figure I the brackets) and how many experienced them after the second dose (the second figure in the brackets) of the vaccine.
• fatigue: younger group (47.4 per cent vs 59.4 per cent) compared to older group (34.1 per cent vs 50.5 per cent )
• headache: younger group (41.9 per cent vs 51.7 per cent) compared to older group (25.2 per cent vs 39.0 per cent)
• muscle pain: younger group (21.3 per cent vs 37.3 per cent) compared to older group (13.9 per cent vs 28.7 per cent)
• chills: younger group (14.0 per cent vs 35.1 per cent) compared to older group (6.3 per cent vs 22.7 per cent)
• joint pain: younger group (11.0 per cent vs 21.9 per cent) compared to older group (8.6 per cent vs 18.9 per cent)
• fever: younger group (3.7 per cent vs 15.8 per cent) compared to older group (1.4 per cent vs 10.9 per cent)
• vomiting: reported less frequently in the older group and was similar after either dose
• diarrhoea: reported less frequently in the older group and was similar after each dose.
The dossier also showed the vaccine provides strong protection against COVID-19 even after a single dose.

NOTE: The Press Council has decided that this article breached its Standards of Practice. Read the full adjudication here.
More Coverage
Originally published as Allergy warning over Pfizer COVID vaccine





