Fast track hopes for SA vaccine to block COVID-19’s track to lungs
A coronavirus vaccine candidate developed by Flinders researchers with supercomputers and AI is now in animal trials with hopes of human trials within weeks.

Coronavirus News
Don't miss out on the headlines from Coronavirus News. Followed categories will be added to My News.
A South Australian breakthrough vaccine candidate against COVID-19 could be ready for limited public use as early as August if proved safe and effective.
A team headed by Flinders University Professor Nikolai Petrovsky, chairman and research director at local company Vaxine Pty Ltd, with a long and celebrated history of developing pandemic vaccines, is working with artificial intelligence to squeeze “15 years of work into 15 weeks”.
The vaccine has gone into animal trials in the United States, with the results expected in six to eight weeks, and subsequent human trials are already being organised to follow within weeks if it proves safe in animals and is approved by authorities.
Volunteers would receive two doses over about four weeks, then researchers would wait another three weeks to assess the results.

Prof Petrovsky said it was then up for regulatory and government review.
“If it does prove safe and effective it could be allowed for emergency use in frontline workers such as doctors and nurses, or it could be approved for a much larger clinical trial in humans,” he said.
“Or, like the swine flu, it could go directly to the population if preliminary work shows it to be safe and effective. In a pandemic you don’t have the luxury of developing a vaccine over 15 years which is normal, you have to compress it into 15 weeks. I am fairly confident we are going to get some good results, which is why we are already planning the human trials.
MORE NEWS:
Experts challenge Morrison’s lockdown timetable
We’re scoring goals but what’s our pandemic endgame?
“We are not promising a vaccine in four months, but that is our timeline and that is the target we are shooting for.”
Prof Petrovsky is finishing a fortnight of self-isolation at home after returning from the US and says he feels fine.
He is now waiting “with bated breath” for the animal trials to unfold using a platform he has previously used in pandemic vaccine development funded by the US Government.
Prof Petrovsky noted the urgency of the pandemic meant some vaccine candidates were bypassing animal trials but he felt it was important to be assured his vaccine candidate was safe and effective before moving on to humans.
“We are nevertheless trying to do it as fast as humanly possible,” he said.
“You can’t cut corners with safety, but you can cut red tape.
“Fortunately we have been funded by the US Government over the past 15 years to develop pandemic vaccines against things like SARS and ebola, so we have a long track record; we were the first in the world to develop a vaccine against swine flu.”
The latest cloud-based technology provided by Oracle enabled the team to “dramatically speed up our ability to analyse the COVID-19 virus and use this information to design the vaccine candidate”.
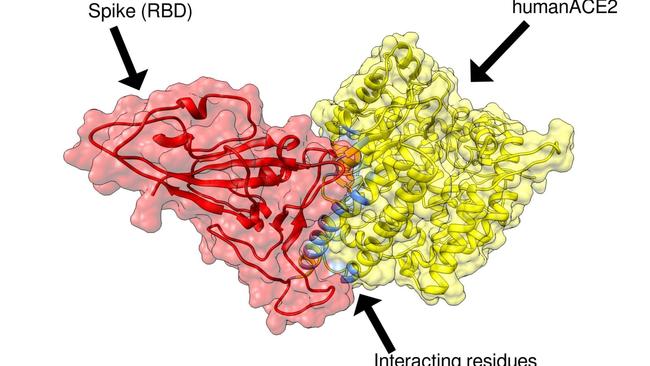
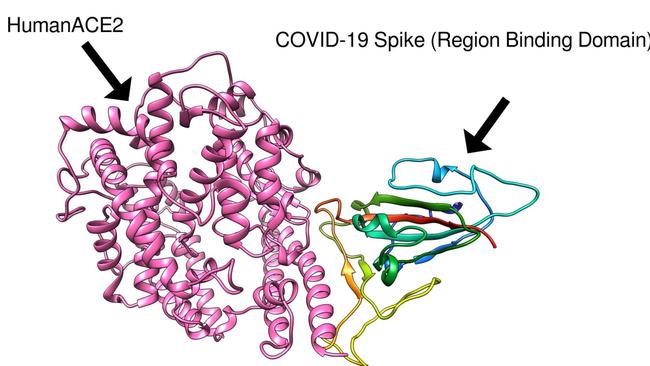
“As soon as the genomic sequence of COVID-19 became available in January, we immediately used this, combined with our previous experience in developing a SARS coronavirus vaccine, to characterise the key viral attachment molecule called the spike protein,” Prof Petrovsky said.
“We used computer models of the spike protein and its human receptor, ACE2, to identify how the virus was infecting human cells, and then were able to design a vaccine to block this process.”
Vaxine’s vaccine mimics the portion of the virus spike protein attaching to human ACE2 receptor, which the virus uses to gain entry into cells lining the human lung.
The aim is to induce human antibodies to be ready to bind to the real COVID-19 spike protein, blocking it from binding to ACE2 and thereby preventing infection.
Flinders University Associate Professor Dimitar Sajkov, a respiratory physician, has been involved in previous human trials of the team’s pandemic vaccines and hopes to similarly lead clinical trials of this new COVID-19 vaccine candidate.
The team had exploited the latest technologies, super computers and AI, to shave years off normal development times, he said: “We achieved great results with Vaxine’s swine flu vaccine developed during the 2009 swine flu pandemic, where we commenced clinical trials of a vaccine within three months of discovery of the virus.”

