Brisbane biotech AnteoTech gets green light for rapid COVID test in Europe
A Brisbane biotech firm has received the green light to roll out its COVID-19 rapid test in Europe over the next few months.
Business
Don't miss out on the headlines from Business. Followed categories will be added to My News.
Brisbane biotech firm AnteoTech will roll out its rapid COVID-19 test in Europe over the next few months joining fellow Queensland startup Ellume in the fight against the pandemic.
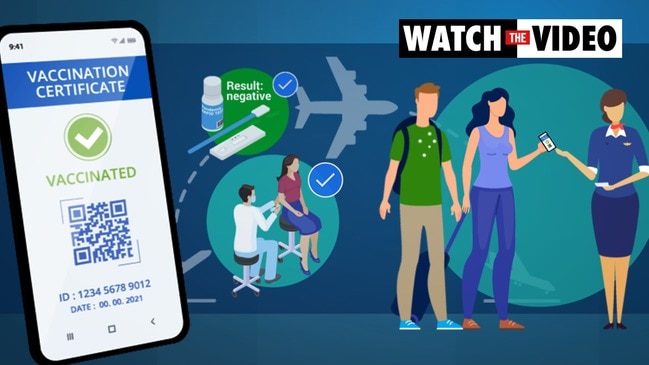
ASX-listed AnteoTech said Monday it had received CE mark registration for the test that can detect the virus within 15 minutes and process up to 60 patients an hour.
AnteoTech said it had manufacturing facilities in Spain ready to start making the testing kits from later this month as borders open up across Europe.
AnteoTech is one of the first Australian companies to receive European regulatory approval for a rapid COVID-19 test, allowing it to immediately enter the market of 447 million people.
CE marking indicates conformity with health, safety, and environmental protection standards for products sold within the European Economic Area and the UK.
AnteoTech’s technology, which detects the virus using a molecular surface coating ‘glue,’ is already used in Ellume’s test which recently received US Federal Drug Administration approval to roll out in the world’s biggest economy.
Brisbane-based Ellume won a $US235m contract with the US Department of Defence in February for the manufacture and distributuon of its rapid COVID-19 test across the armed forces.
The cash followed the US National Institute of Health granting the company $US30m to ramp up production.
AnteoTech chief executive Derek Thomson said the European regulatory approval was a key milestone in the company’s strategy to build a global market for its products.
“We believe we have a superior test with high sensitivity,” said Mr Thomson. “We can be ready to start production in April from facilities in Spain. It will allow us to capture a share of the growing and evolving antigen rapid testing market in Europe and the UK.”
Mr Thomson said there would be increasing demand for testing kits to screen people travelling across borders that were now opening up across Europe.
“We see a lot of interest from airlines that will need to screen a lot of people quickly,” he said. “You can turn up at an airport, be tested and then you would be ready to go.”
The company also planned to supply the testing kits to hospitals, doctor’s surgeries and health clinics across Europe.
Mr Thomson said the company’s small team of 28 people based in the Brisbane suburb of Eight Mile Plains had been able to commercialise the test kit within a short time helped by a $1.4m State Government grant under its essential goods and supply chain program.
He said the company also was ready to manufacture the kits from Brisbane to supply the domestic market and the Asia Pacific.

“We don’t see COVID-19 going away anytime soon so there will be demand for this technology in the future,” he said. “We have shown that Brisbane has the skills set to produce this sort of technology in a rapid time-frame.”
AnteoTech’s tests also are able to diagnose other illnesses including influenza and sepsis, a condition that involves the body’s extreme and life threatening response to infection.
“The delivery of effective lifesaving treatment for sepsis is time critical,” he said. “It is difficult to diagnose sepsis promptly and it is often missed resulting in a very high global mortality rate beyond that of COVID-19 today.”
Queensland Treasurer and Minister for Investment Cameron Dick said Queensland ingenuity was proving crucial in stopping the spread of COVID-19.
“This tick from Europe will help AnteoTech accelerate commercialisation of their product in Australia, generating more than $2 million in private sector investment,” said Mr Dick. AnteoTech shares climbed 6 per cent to 26 cents yesterday.
Originally published as Brisbane biotech AnteoTech gets green light for rapid COVID test in Europe


